The Ion Product Constant for Water (Kw)
advertisement

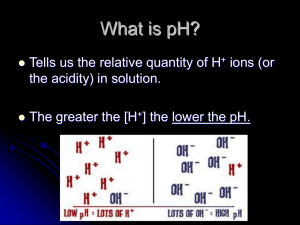
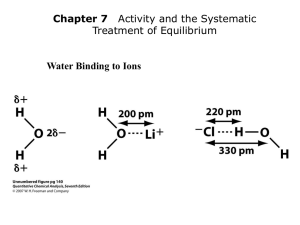
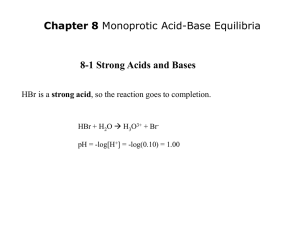
The Ion Product Constant for Water (Kw) • Pure water dissociates according to the following reaction: H2O(l) H+(aq) + OH-(aq) • There is an equal amount of H+ and OH- ions in solution (neutral, pH = 7) • at 25°C [H+] = [OH-] = 1x10-7 mol/L • equilibrium constant for the dissociation of water: Kw Kw = [H+][OH-] = (1x10-7)(1x10-7) = 1x10-14 * small k, reactants are favoured (does not go to completion) Since strong acids and bases dissociate completely in water, [H+] = [acid] @ 25°C acids: [H+] > [OH-] [H+] > 1x10-7 [OH-] < 1x10-7 bases: [OH-] > [H+] [H+] < 1x10-7 [OH-] > 1x10-7 We can use Kw to calculate [H+] and [OH-] in solutions Ex 1) Find the [H+] and [OH-] in: (a) 2.5 M nitric acid (b) 0.16 M Barium hydroxide (a) C HNO3 2.5 M H+ 2.5 M Kw = [H+][OH-] (b) C Ba(OH)2 0.16 M Kw = [H+][OH-] + NO3- [OH-]= Kw / [H+] = (1x10-14)/(2.5) = 4x10-15 M Ba2+ + 2 OH0.32 M [H+] = Kw / [OH-] = (1x10-14)/(0.32) = 3.1x10-14 M pH and pOH pH: The Power of the Hydronium Ion • A measure of the amount of H+ ions in a solution • Convenient way to represent acidity since [H3O+] is usually a very small number • 2 factors determine pH • ionization • concentration because they both contribute to the number of H+ or OH- molecules in a solution. • The practical scale goes from 0 14 pH = -log [H3O+] pOH = -log [OH-] In neutral water, pH = -log [H3O+] = -(log(1 x 10-7) = 7 pOH = -log [OH-] = -(log(1 x 10-7) = 7 Note: pH + pOH = 14, always, regardless of solution! Another way to calculate [H3O+] & [OH-] in solution: [H3O+] = 10-pH [OH-] = 10-pOH Ex) A liquid shampoo has a [OH-] of 6.8x10-5 mol/L (a) Is the shampoo acid, basic or neutral? (b) What is [H3O+]? (c) What is the pH and pOH of the shampoo? (a) [OH-] = 6.8x10-5 > 1.0x10-7, basic (b) [H3O+] = Kw / [OH-] = (1.0x10-14)/(6.8x10-5) = 1.5x10-10 mol/L (c) pH = -log [H3O+] pOH = -log [OH-] = -log [1.5x10-10] = -log [6.8x10-5] = 9.83 = 4.17 check: 9.83 + 4.17 = 14 ! HOMEWORK P382 #1-4 p390 #9-12 p392 #13-18