Step 2
advertisement

Harris: Quantitative Chemical Analysis, Eight Edition
CHAPTER 07:
ACTIVITY AND THE
SYSTEMATIC
TREATMENT OF
EQUILIBRIUM
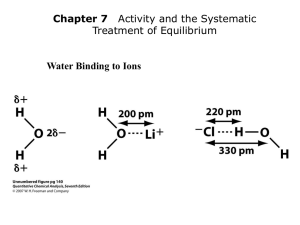
Ionic and hydrated radii of several ions
Water Binding to Ions
Equilibrium Constants
with Concentrations and Activities
Fe3+ + SCN- = Fe(SCN)2+
Pale yellow Colorless
Red
7-1 The Effect of Ionic Strength on Solubility of Salts
CaSO4(s) = Ca2+ + SO42-
Ksp = 2.4 X 10-5
(8-2)
When we add salt to a solution, we say that the ionic strength of the solution
increases.
We call this region the ionic atmosphere (Figure 8-2).
The greater the ionic strength of a solution, the higher the charge in the
ionic atmosphere. Each ion-plus-atmosphere contains less net charge and
there is less attraction between any particular cation and anion.
The effect is to reduce their tendency to come together, thereby increasing the
solubility of CaSO4.
Thiocyanate
Phenol
Potassium hydrogen tartrate
Phenolate
Ionic strength, µ, is a measure of the total concentration of ions in solution.
Ionic strength:
Box7-1 Salts with Ions of Charge ≥ |2| Do Not Fully Dissociate
Ion pair formation constant: Mn+(aq) + Lm-(aq) = Mn+Lm-(aq)
Ion pair
7-2 Activity Coefficients
To account For the effect of ionic strength, concentrations are replaced
by activities:
Activity of C:
The activity of species C is its concentration multiplied by its activity
coefficients.
General form of
equilibrium constant:
Ksp = ACa2+ASO42- = [Ca2+]γCa2+[SO42-]γSO42-
The ionic atmosphere model leads to the extended Debye-Hückel equation,
relating activity coefficients to ionic strength:
Extended Debye-Hückel
equation:
To find activity coefficients for ionic strengths above 0.1 M (up to molalities
of 2-6 mol/kg for many salts), more complicated Pitzer equations are usually
used.
In linear interpolation, we assume that values between two entries of a
table lie on a straight line.
Interpolation:
7-3 pH Revisited
pH = -logAH+ = -log[H+]γH+
(8-8)
When we measure pH with a pH meter, we are measuring the negative
logarithm of the hydrogen ion activity, not its concentration.
However, the concentration of H+ in 0.10 M KCl (1.26 X 10-7 M) is 26%
greater than the concentration of H+ in pure water (1.00 X 10-7 M).
7-4 Systematic Treatment of Equilibrium
The systematic treatment of equilibrium is a way to deal with all types of
chemical equilibria, regardless of their complexity.
The charge balance is an algebraic statement of electroneutrality: The sum
of the positive charges in solution equals the sum of the negative charges in
solution.
[H+] + [K+] = [OH-] + [H2PO4-] + 2[HPO42-] + 3[PO43-]
(8-11)
The coefficient in front of each species always equals the magnitude of the
charge on the ion.
[H+] = 5.1 X 10-12 M
[K+] = 0.0550 M
[OH-] = 0.0020 M
[H2PO4-] = 1.3 X 10-6 M
[HPO42-] = 0.0220 M
[PO43-] = 0.0030 M
[H+] + [K+] = [OH-] + [H2PO4-] + 2[HPO42-] + 3[PO43-]
5.1 X 10-12 + 0.0550 = 0.0020 + 1.3 X 10-6 + 2(0.0220) + 3(0.0030)
0.0550 M = 0.0550 M
Charge balance:
Where [C] is the concentration of a cation, n is the charge of the cation, [A]
is the concentration of an anion, and m is the magnitude of the charge of the
anion.
The mass balance, also called the material balance, is a statement of the
conservation of matter. The mass balance states that the quantity of all
species in a solution containing a particular atom (or group of atoms) must
equal the amount of that atom (or group) delivered to the solution.
CH3CO2H = CH3CO2- + H+
Acetic acid
Acetate
Mass balance for
0.050M = [CH3CO2H] + [CH3CO2-]
Acetic acid in water: What we put into Undissociated Dissociated
the solution
product
product
0.0250 M = [H3PO4] + [H2PO4-] + [HPO42-] + [PO43-]
K
La(IO3)3(s) =sp La3+ + 3IO3Iodate
[IO3-] = 3[La3+]
[Total iodate] = 3[total lanthanum]
[IO3-] + [LaIO32+] = 3{[La3+] + [LaIO32+] + [LaOH2+]}
Box 7-2 Calcium Carbonate Mass Balance in Rivers
CaCO3(s) + CO2(aq) + H2O = Ca2+ + 2HCO3Calcite
Bicarbonate
(A)
7-5 Applying the Systematic Treatment of Equilibrium
A simple Example: Ionization of Water
Step 1 Pertinent reactions. The only one is Reaction 8-13.
Step 2 Charge balance. The only ions are H+ and OH-, so the charge balance is
[H+] = [OH-]
(8-14)
Step 3 Mass balance. Reaction 8-13 creates one H+ for each OH-. The mass
balance is simply [H+] = [OH-], which is the same as the charge balance for
this system.
Step 4 Equilibrium constant expression.
KW = [H+]γH+[OH-]γOH- = 1.0 X 10-14
(8-15)
This is the only step in which activity coefficients enter the problem.
Step 5 count equations and unknowns. We have two equations,
8-14 and 8-15, and two unknowns, [H+] and [OH-].
Step 6 Solve.
[H+]γH+[OH-]γOH- = 1.0 X 10-14
[H+] · 1 · [H+] · 1 = 1.0 X 10-14
[H+] = 1.0 X 10-7 M
pH = -logAH+ = -log[H+]γH+ = -log(1.0 X 10-7)(1) = 7.00
Solubility of Calcium Sulfate
Step 1 Pertinent reactions. Even in such a simple system, there are quite a
few reactions:
There is no way you can be expected to come up with all of these
reactions, so you will be given help with this step.
Step 2 Charge balance. Equating positive and negative charges gives
2[Ca2+] + [CaOH+] + [H+] = 2[SO42-] + [HSO4-] + [OH-]
(8-21)
Step 3 Mass balance. Reaction 8-16 produces 1mole of sulfate for each mole
of calcium. No matter what happens to these ions, the total concentration of
all species with sulfate must equal the total concentration of all species with
calcium:
[Total calcium] = [total sulfate]
[Ca2+] + [CaSO4(aq)] + [CaOH+] = [SO42-] + [HSO4-] + [CaSO4(aq)] (8-22)
Step 4 Equilibrium constant expressions. There is one for each chemical
reaction.
Step 4 is the only one where activity coefficients come in.
Step 5 Count equations and unknowns. There are seven equations (8-21
through 8-27) and seven unknowns: [Ca2+], [SO42-], [CaSO4(aq)],
[CaOH+], [HSO4-], [H+], and [OH-]. In principle, we have all the
information necessary to solve the problem.
Step 6 Solve. Well, this is not easy! We don’t know the ionic strength, so
we cannot evaluate activity coefficients. Also, where do we start when
there are seven unknowns?
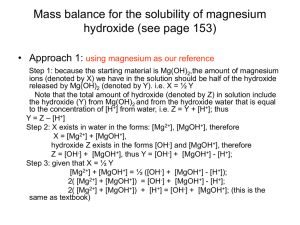
Solubility of Magnesium Hydroxide
Step 1 Pertinent reactions are listed above.
Step 2 Charge balance: 2[Mg2+] + [MgOH+] + [H+] = [OH-]
Step 3 Mass balance. This is a little tricky. From Reaction 8-30, we could
say that the concentrations of all species containing OH- equal two times
the concentrations of all magnesium species. However, Reaction 8-32 also
creates 1 OH- for each H+. The mass balance accounts for both sources of
OH-:
[OH-] + [MgOH+] = 2{[Mg2+] + [MgOH+]} + [H+]
Species contatining OH-
(8-34)
Species containing Mg2+
After all this work, Equation 8-34 is equivalent to Equation 8-33.
Step 4 Equilibrium constant expressions are in Equations 8-30 through 832.
Step 5 Count equations and unknowns. We have four equations (8-30 to 833) and four unknowns: [Mg2+], [MgOH+], [H+], and [OH-].
Step 6 Solve.
2[Mg2+] + [MgOH+] = [OH-]
2[Mg2+] + K1[Mg2+][OH-] = [OH-]
(8-35)