Chapter 7
Acids and Bases
Fig. 9-CO, p. 251
Bronsted-Lowry Definition
B A A D
a
s
e
c
c
e
p
t
s
c
i
d
o
n
a
t
e
s
The hydrogen ion
is a proton
+
H
(base)
(acid)
H2O + HCl
H3O+ +
Cl-
Brønsted-Lowry Acids & Bases
• We can use curved arrows to show the transfer of a
proton from acetic acid to ammonia:
: O:
H
H
:
:
+
:N H
H
A ce ti c aci d
A m m on i a
(p ro to n d o n o r) (p ro ton ac ce p to r)
CH 3 - C-O:
:
:
CH 3 - C-O H
: O:
A ce tate i on
+
+
H N H
H
A m m on i u m
io n
H2O +
NH3
OH- + NH4+
OH- + NH4+
H2O +
NH3
H2O +
(acid)
NH3
(base)
OH- + NH4+
(base)
(acid)
9 Brønsted-Lowry Acids & Bases
con ju g ate aci d -b as e p ai r
con ju g ate aci d -b as e p ai r
CH 3 CO O H
A ce ti c aci d
(aci d )
© 2006 Thomson Learning, Inc.
All rights reserved
+
-
CH 3 CO O
A ce tate
io n
A m m o nium
io n
(b as e )
(c on ju g ate b as e
ace ti c ac i d )
(co n ju g ate aci d
of am m o n i a)
+
N H4
+
N H3
A m m on i a
9-8
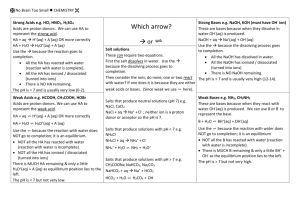
Identify the Acid and the Base in
Each Chemical Equation
+
O
• HBr + H2O → H3
+ Br
• H2O + CN → HCN + OH
+
←
• HF + H2O → H3O + F
Acid-Base Equilibria
• HCl is a strong acid, which means that the position of
this equilibrium lies very far to the right.
H Cl + H 2 O
Cl
-
+ H3 O +
• In contrast, acetic acid is a weak acid, and the position
of its equilibrium lies very far to the left.
CH 3 COOH + H 2 O
A ce ti c aci d
CH 3 COO
-
A ce tate i o n
+ H3 O
+
Amphoteric
A substance whose
ability to behave as an
acid is about the same
as its ability to behave
as a base.
-
For pure water…
[
OH- ] = 0.0000001 M
= 10-7 M
+ = 0.0000001 M
H
O
[ 3 ]
= 10-7 M
RULE
[
OH
] [ H3O+]
10-7 x 10-7
0.0000001 M
= Kw
= 10-14
X 0.0000001 M =
0.00000000000001 M
• The equation for the ionization of water applies not
only to pure water but also to any aqueous solution.
• The product of [H3O+] and [OH-] in any aqueous
solution is equal to 1.0 x 10-14.
• For example, if we add 0.010 mol of HCl to 1.00 liter of
pure water, it reacts completely with water to give 0.010
mole of H3O+.
• In this solution, [H3O+] is 0.010 or 1.0 x 10-2.
• This means that the concentration of hydroxide ion is:
-
[ OH ] =
1.0 x 10 - 14
1.0 x 10 - 2
= 1.0 x 10 - 1 2
9
© 2006 Thomson Learning, Inc.
All rights reserved
9-19
pH and pOH
• Because hydronium ion concentrations for most
solutions are numbers with negative exponents, we
commonly express these concentrations as pH, where:
pH = -log [H3O+]
• We can now state the definitions of acidic and basic
solutions in terms of pH:
• Acidic solution: one whose pH is less than 7.0.
• Basic solution: one whose pH is greater than 7.0.
• Neutral solution: one whose pH is equal to 7.0.
7
10
log
=
7
-5
log 10
= -5
-2
log 0.01 = 10 = -2
Antilog 5 = 100,000
-9
10
Log 1.4 x
=
Antilog 5.3 =
What is the pH of a
solution with a hydronium
-9
ion conc. of 3.2 x 10 M?
pH = -log [ H3
= -log
+
O
[3.2 x
= - (-8.5)
= 8.5
]
-9
10
]
What is the pH of a
solution with a
hydroxide ion conc.
of .001 M?
The pH of a solution
is 10.0.
What is the [H3O+]
and the [OH-]?
A buffer solution
contains at least two
components:
A component to neutralize
any incoming base
A component to neutralize
any incoming acid
pH Buffers
• The most common buffers consist of approximately
equal molar amounts of a weak acid and a salt of the
weak acid; that is, approximately equal molar amounts
of a weak acid and a salt of its conjugate base.
• For example, if we dissolve 1.0 mole of acetic acid and
1.0 mole of its conjugate base (in the form of sodium
acetate) in water, we have an acetate buffer.
p. 272
pH Buffers
• The effect of a buffer can be quite dramatic
• Consider a phosphate buffer prepared by dissolving
0.10 mole of NaH2PO4 (a weak acid) and 0.10 mole of
Na2HPO4 (the salt of its conjugate base) in enough
water to make 1 liter of solution.
pH
w ate r 7.0
0.10 M p h os p h ate b u ff e r 7.21
p H af te r
ad d i ti o n o f
0.010 m o l e H Cl
p H af te r
ad d i ti o n o f
0.010 m ol e N aO H
2.0
12.0
7.12
7.30
• The average pH of
human blood is 7.4.
• any change greater than
0.10 pH unit in either
direction can cause
illness.
• Acidosis and Alkalosis
Describe condtions
when the pH of blood is
too high or too low.
Chemical Connections 9D, p. 277
Salt
An ionic compound formed
from the reaction between
an acid and a base.
Neutralization
The reaction between an
acid and a base to give a
salt and water.
Salt formation
HCl + NaOH → NaCl + H2O
(acid)
(base)
(salt)
(water)
Hydrogen
chloride
Sodium
hydroxide
Sodium chloride
(acid)
(base)
(salt)
Water