Kw of Water
advertisement

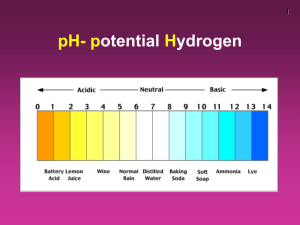
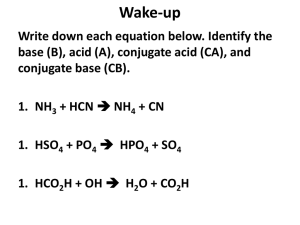
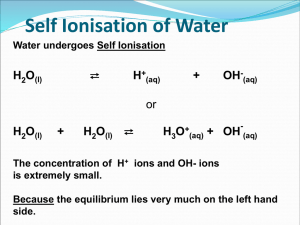
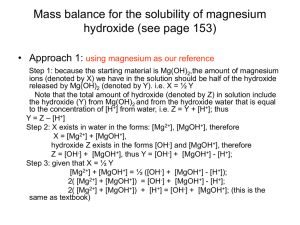
Kw of Water Chapter 16 part III Kw of Water •From the auto-ionization expression of water we get the equilibrium constant expression. •2 H2O H3O+ + OH•Kw= [H3O+ ][OH-] •Why not H2O? •Kw= [H+ ][OH-] Kw is the ion-product Kw is aka dissociation constant of water It has been found experimentally that at 25 °C, [H+ ]=[OH-] and they both equal 1X10-7M Since Kw= [H+ ][OH-] = [1X10-7M]2 Then Kw = 1X 10-14. What does this mean? This means that any aqueous solution at 25 ° C, no matter what it (the water) contains, the product of [H+] and [OH-] MUST always equal 1.0 X 10-14. There are 3 possibilities: A neutral solution where [H+]=[OH-]. An acid solution where [H+]>[OH-]. A basic solution where [H+]<[OH-]. In all 3 of the situations: = [H+][OH-] = 1.0 X 10-14. So in any given aqueous situation, one may calculate the [H+] or [OH-] as required for any solution at 25°C. State if Acidic, Basic or Neutral. A. 1.0X10-5 M OH- 1.0 X10-9 M H+ B. 1.0X10-7 M OH- 1.0 X10-7 M H+ C. 1.0X10-15 M OH- 10.0 M H+ Kw Answers A. Basic B. Neutral C. Acid How to Solve for the [ions] Kw = [H+][OH-] 1.0 X 10-14= [H+][OH-] [H+] = [OH-]/(1.0 X 10-14) Kw varies with Temperature if the Kw at 60° C is 1.0 X 10-13 Then, using Le Châtlier’s principle predict whether the ionization of water is endo or exothermic. • 2 H2O H3O+ + OH• Kw increases • from 1.0 X 10-14 to 1.0 X 10-13 • Therefore the system adjusted to form more product in the presence of heat. • This indicates the reaction is endothermic So Example Calculate the [OH-] and [H+] at 60° C in a neutral solution. Neutral means what about [H+] to [OH-]? Answer: [H+] = [OH-] So: Kw= [H+] x [OH-] = 1.0 X 10-13 [H+] = [OH-] = (1.0 X 10-13 )½ =3.0 X 10-7 M pH scale is an easy way to represent acidity. pH = -log[H+] At a neutral solution at 25 °C [H+] = [OH-] = (1.0 X 10-14)½ = 1.0 X 10-7 What is the pH of this? pH = -log[H+] = -log(1.0 X 10-7) Take out you calculator and what do you get? 7.00 Sig Figs in Log problems The number of sig figs in an original number equals the number of decimal places in the pH. Example: If sample is Kw= [H+] = 1.0 X 10-7 How many sig fig? 2 This pH is … 7.00 2 decimal places for the two sig figs. pH vs. pOH If pH is = -log [H+] Then pOH = -log [OH-] And pK = -log K Note that pH changes by 1 for every power of 10 in the change of concentration. Examples Calculate the pH and pOH of each 1.0 X 10-3 M OH pOH = 3.00 pH = 11.00 H+ = Kw/[OH-] = (1.0 X 10-14)/(1.0 X 10-3) = 1.0 x 10-11 1.0 M H+ pH = 0.00 [OH-]=Kw/[H+]= 1.0x10-14/1 pOH = 14 Remember Kw =[H+][OH-] And therefore, -log K = -log [H+] + -log [OH-] log K = log[H+] + log[OH-] pKw = pH = pOH At 25 °C pKw = 14.00 (1.0 X 10-14) Thus pH = pOH = 14 at 25 °C Summary of General Strategies Think Chemistry: focus on solution and the components. It is usually easy to identify one reaction that is not important. Be systematic: Acid-base problems require step by step approach. Be Flexible: Although all acid-base problems are similar, important differences do occur. Do not force a given problem into matching a problem you have solved before. Summary of General Strategies Be patient: the complete solution to a complicated problem cannot be seen immediately in all its detail. Pick the problem apart into workable steps. Understand & Think: don’t just memorize.