pH - christophersonbiology
advertisement
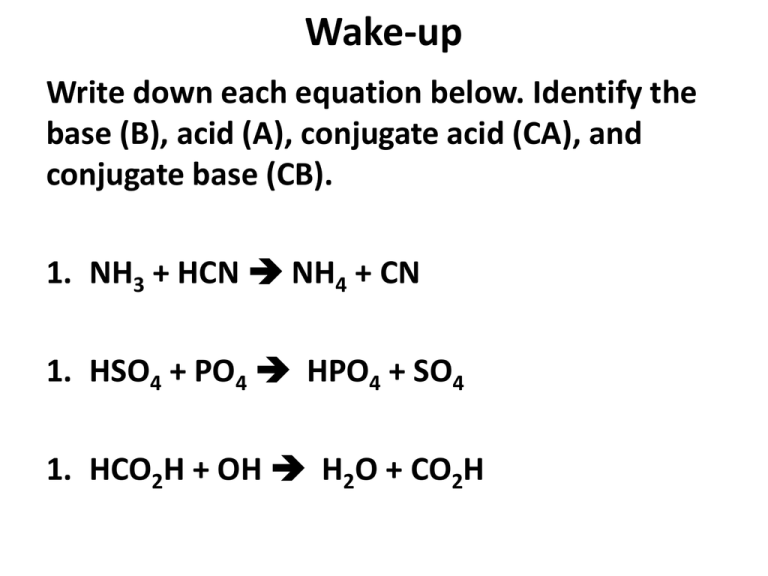
Wake-up Write down each equation below. Identify the base (B), acid (A), conjugate acid (CA), and conjugate base (CB). 1. NH3 + HCN NH4 + CN 1. HSO4 + PO4 HPO4 + SO4 1. HCO2H + OH H2O + CO2H pH and pOH Ways to measure Acidity/Basicity What is pH? What is pOH? What does pH represent? Parts Hydrogen pH Measurement that reveals if a solution is acidic or basic What is the pH Scale? Runs from 0.0 to 14.0 with 7.0 being neutral; Logarithmic pH scale Neutral Acid 1 Base (Alkaline) 6 7 8 14 Logarithmic Scale A ten-fold difference between each successive full number on the scale Logarithmic Scale – ACID Each whole pH value BELOW 7 is ten times more acidic than the next higher value. For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. Logarithmic Scale: BASE Each whole pH values ABOVE 7 is ten times more basic than the next lower whole value. For example, pH 10 is ten times more alkaline than pH 9 and 100 times (10 times 10) more alkaline than pH 8. Basic Equations (Reference Table) pH + pOH = 14 pH = -log[H+] pOH = -log[OH-] [H+] = 10-pH [OH-] = 10-pOH Example #1 What is the pH of a 0.031 M HCl solution? Known = 0.031 M HCl Unknown = pH pH = -log[H+] pH = -log [0.031M] pH = 1.5 Example #2 What is the pH of a 2.50 x 10-6 M HNO3 solution? Known = 2.50 x 10-6 M HNO3 Unknown = pH pH = -log[H+] pH = -log [2.50 x 10-6 M] pH = 5.60 Example #3 What is the pH of a 0.025 M NaOH solution? Known = 0.025 M NaOH Unknown = pH pOH = -log[OH+] pOH = -log [0.025 M] = 1.6 pOH pH + pOH = 14 pH + 1.6 = 14 pH = 12.4 Example #4 What is the pH of a 0.000051 M NaOH solution? Known = 0.000051 M NaOH Unknown = pH pOH = -log[OH+] pOH = -log [0.000051 M] = 4.3 pOH pH + pOH = 14 pH + 4.3 = 14 pH = 9.7 Calculating pOH • pOH = 14 – pH • pOH = -log[OH-] EXAMPLE: What is the pOH of a solution that has a hydroxide ion concentration of 4.82 x 10-5 M ? pOH = - log [OH-] pOH = - log [4.82 x 10-5] pOH = 4.32 YOU TRY! • Find the pH of the solution if [H+] = 1 x 10-4 M pH = 4 • What is the hydrogen ion concentration if pH = 7.1? 7.1 = -log [H+] 10 – 7.1 = [H+] = 7.9 x 10-8 M • What is the pH if [OH-] = 4 x 10 -11 M? pOH = -log [OH-] pOH = -log [4 x 10 -11 ] = pH scale Neutral Acid 1 Strong Acid Base 6 7 8 Weak Acid Weak Base 14 Strong Base Breakdown of water















![[H3O+], [OH–], pH - BC Learning Network](http://s2.studylib.net/store/data/005610503_1-47ff8b2322e271c154ebb4db3f00887e-300x300.png)