File
advertisement

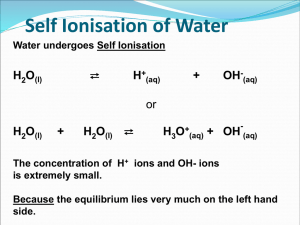
Self Ionisation of Water Water undergoes Self Ionisation ⇄ H2O(l) H+(aq) OH-(aq) + or H2O(l) + H2O(l) ⇄ H3O+(aq) - + OH (aq) The concentration of H+ ions and OH- ions is extremely small. Because the equilibrium lies very much on the left hand side. Show how [H+] = 1.0 X 10-7 • Degree of ionisation is extremely small • Kw = Kc[H2O]= [H+][OH-] = = 1 x 10-14 (at 25ºC) • Kw is the Ionic Product of water/dissociation product of water • Kw is temperature dependent ( not pressure or concentration dependent) • Increase temperature will increases the ionic product ( no effect on pH of water though) • Acidic solution [H+] greater [OH-] • Pure Water is a very very weak electrolyte • ( only 1 in every 600 million water molecules ionise) Kw is temperature dependent T (°C) Kw (mol2/litre2) 0 0.114 x 10-14 10 0.293 x 10-14 20 0.681 x 10-14 25 1.008 x 10-14 30 1.471 x 10-14 40 2.916 x 10-14 50 5.476 x 10-14 Kw of pure water increases as the temperature increases The ionic product of water is the product of the hydrogen and hydroxide ion concentration in 1litre of water at 25 °C Kw = [H+][OH-] = 1 × 10-14 at 25 °C pH The ionic product of water is the product of the hydrogen and hydroxide ion concentration in 1litre of water at 25 °C Kw = [H+][OH-] = 1 × 10-14 at 25 °C [H+ ] x [OH- ] = 1 x 10-14 = [1 x 10-7 ] x [1 x 10-7 ] [H+ ] of water is at 250C is 1 x 10-7 mol/litre Replacing [H+ ] with pH to indicate acidity of solutions pH 7 replaces [H+ ] of 1 x 10-7 mol/litre where pH = - Log10 [H+ ] pH At 250C Kw = 1 x 10-14 mol2/litre2 [H+ ] x [OH- ] = 1 x 10-14 mol2/litre2 This equilibrium constant is very important because it applies to all aqueous solutions - acids, bases, salts, and non-electrolytes - not just to pure water. pH of Common Substances Acidic Neutral Basic 9 The pH Scale • Each pH unit is 10 times as large as the previous one • A change of 2 pH units means 100 times more basic or acidic x10 x100 Limitations 1. Doesn’t cover very HIGH concentration (pH above 10-1) or very low pH values (pH below 10-14) 2. Must be aqueous 3. Affected by temperature ( standard temperature is 25°C) The ionic product of water is the product of the hydrogen and hydroxide ion concentration in 1litre of water at 25 °C Acid–Base Concentrations in Solutions concentration (moles/L) 10-1 OH- H+ 10-7 H+ OH- OH- H+ 10-14 [H+] > [OH-] [H+] = [OH-] acidic solution neutral solution [H+] < [OH-] basic solution pH Scale Soren Sorensen (1868 - 1939) The pH scale was invented by the Danish chemist Soren Sorensen to measure the acidity of beer in a brewery. The pH scale measured the concentration of hydrogen ions in solution. The more hydrogen ions, the stronger the acid. The pH Scale 1 2 Strong Acid 3 4 Weak Acid 5 6 7 Neutral 8 9 10 11 Weak Alkali 12 13 14 Strong Alkali pH Scale The quantity of hydrogen ions in solution can affect the color of certain dyes found in nature. These dyes can be used as indicators to test for acids and alkalis. An indicator such as litmus (obtained from lichen) is red in acid. If base is slowly added, the litmus will turn blue when the acid has been neutralized, at about 6-7 on the pH scale. Other indicators will change color at different pH’s. A combination of indicators is used to make a universal indicator. Measuring pH • Universal Indicator Paper • Universal Indicator Solution • pH meter pH scale [H+] > 10-7M, pH < 7 ACIDIC [H+] < 10-7M, pH > 7 BASIC [H+] = 10-7M, pH = 7 NEUTRAL The larger the hydrogen Ion concentration The smaller the pH, The stronger the acid The pH Scale The pH Scale 17 • Each pH unit is 10 times as large as the previous one • A change of 2 pH units means 100 times more basic or acidic x10 x100 Limitations 1. Doesn’t cover very HIGH concentration (pH above 10-1) or very low pH values (pH below 10-14) 2. Must be aqueous 3. Affected by temperature ( standard temperature is 25°C) pH is temperature dependent T (°C) pH 0 7.12 10 7.06 20 7.02 25 7 30 6.99 40 6.97 pH of pure water decreases as the temperature increases A word of warning! If the pH falls as temperature increases, does this mean that water becomes more acidic at higher temperatures? NO! Remember a solution is acidic if there is an excess of hydrogen ions over hydroxide ions. In the case of pure water, there are always the same number of hydrogen ions and hydroxide ions. This means that the water is always neutral - even if its pH change pH= 7 at 25° C pH = -Log10 [H+] Defined as the negative log to the base 10 of the molar Hydrogen ion concentration in an aqueous solution pH of bases: pOH pOH= -log10 [OH-] pH + pOH = 14 pH= 14 - pOH pH Exercises c) pH of solution where [H +] a)pH of 0.02M HCl is 7.2x10-8M + pH = – log10 [H ] pH = – log10 [H+] = – log10 [0.020] = – log10 [7.2x10-8] = 1.6989 = 7.14 = 1.70 (slightly basic) b)pH of 0.0050M NaOH pOH = – log10 [OH–] = – log10 [0.0050] = 2.3 pH = 14 – pOH = 14 – 2.3 =11.7 pH Calculations pH pH = -log10[H+] [H+] [H+] = 10-pH [H+] [OH-] = 1 x10-14 pH + pOH = 14 pOH pOH = -log10[OH-] [OH-] [OH-] = 10-pOH pH of dilute aqueous solutions of acids monoprotic e.g. HCl, HNO3 HA(aq) 0.3 M H1+(aq) + A1-(aq) 0.3 M 0.3 M pH = ? pH = - log10 [H+] pH = - log10[0.3M] pH = diprotic e.g. H2SO4 H2A(aq) 0.3 M 2 H1+(aq) + A2-(aq) 0.6 M 0.3 M 0.52 pH = - log10[H+] pH = - log10[0.6M] pH = 0.22 What is the pH of a 0.1 molar soltion of NaOH (careful) What is the pH of 0.05 molar solution of Co(OH)2 ( assume its fully dissociated ) Solving for [H+] • A solution has a pH of 8.5. What is the Molarity of hydrogen ions in the solution? pH = - log [H+] 8.5 = - log [H+] Strong and Weak Acids/Bases Strong acids/bases – 100% dissociation into ions HCl HNO3 H2SO4 NaOH KOH Weak acids/bases – partial dissociation, both ions and molecules CH3COOH NH3 Need to know equilibrium constant pH calculations for Weak Acids and Weak Bases [H+]= √ka×Macid [OH-]= √kb×Mbase For Weak Acids pH = -Log10 For Weak Bases pOH = Log10 pH = 14 - pOH pH of solutions of weak concentrations Weak Base pH of a 0.2M solution of ammonia with a Kb value of 1.8 x 10-5 pH = 11.2681 • Calculate the pH of a 1 molar ethanoic acid solution that is only 1.4% ionised Acid base indicators • Substances that change colour according to pH of solution • Most are weak acids or bases so must only be added in small amounts. The colour of the dissociated molecule is different to the colour of the undissociated molecule • Some indicators dissociate to form weak bases • InH=In- + H+ • InOH = In+ + OH• Chemical equilibrium alters whether in presence of acid or base Theory of Acid Base Indicators Acid-base titration indicators are quite often weak acids. For the indicator HIn The equilibrium can be simply expressed as HIn(aq, colour 1) Methyl orange H+(aq) + In-(aq, colour 2) •HIn (red, Acid)= H+ + In- (yellow, Base) •In acid: the equilibrium lies to the ______ giving it a ___ colour •In base: the equilibrium lies to the ______ giving it a ___ colour • : dynamic equilibrium: apply a stress by adding or removing H+ ions will shift the equilibrium •The equilibrium will shift depending on whether H+ ions or OH- ions exist. Therefore causing a colour change Draw rough trend graph Name of Indicator Approx Range Methyl Orange 3.1-4.4 Litmus 5-8 Acid Colour Lower pH Base Colour Higher pH yellow red Phenolphth 8.3-10 alein red blue colourless pink Acid Base Titration Curves Strong Acid – Strong Base Weak Acid – Strong Base Strong Acid – Weak Base Weak Acid – Weak Base Choice of Indicator for Titration • Indicator must have a complete colour change in the steepest part of the pH titration curve • Indicator must have a distinct colour change • Indicator must have a sharp colour change Indicators for Strong Acid Strong Base Titration Both phenolphthalein and methyl orange have a complete colour change in the vertical section of the pH titration curve Indicators for Strong Acid Weak Base Titration Methyl Orange is used as indicator for this titration Only methyl orange has a complete colour change in the vertical section of the pH titration curve Phenolphthalein has not a complete colour change in the vertical section on the pH titration curve. Indicators for Weak Acid Strong Base Titration Phenolphthalein is used as indicator for this titration Only phenolphthalein has a complete colour change in the vertical section of the pH titration curve Methyl has not a complete colour change in the vertical section on the pH titration curve. Indicators for Weak Acid Weak Base Titration No indicator suitable for this titration because no vertical section Neither phenolphthalein nor methyl orange have completely change colour in the vertical section on the pH titration curve Page 261, 262 Question NB to practise