Br 2 (g)

Saturday Study Session 1
Theme of the Class
Enthalpy
Session 3 – Delta H Four Different
Ways
Enthalpy. Anyone not in
Chemistry thinks you’re saying Empathy. I feel your pain.
Energy
The capacity to do work or to produce heat.
The Two Types of Energy
Potential: due to position or composition can be converted to kinetic.
Kinetic: due to motion of the object
State Function
Depends only on the present state of the system - not how it arrived there.
It is independent of pathway .
Enthalpy is a state function
It ’ s like going to Vegas. On the way home you know you lost
$400. It doesn ’ t matter what casino or how many bets you placed. You still lost money.
First Law
First Law of Thermodynamics:
The energy of the universe is constant.
If you lose $$ in Vegas, someone else made $$.
Enthalpy
H = energy flow as heat (at constant pressure and volume)
Thus the change of enthalpy is the change in the amount of energy of a system.
A block of wood burning has a negative change in enthalpy.
A tree taking energy from the sun and building a tree has a positive change in enthalpy.
If you lost money in Vegas, that is negative
$.
If you won money, that is positive
$ .
4 ways to calculate change in enthalpy
1. Calorimetry – use q=mc
t to find heat gained or lost by water.
2. Hess ’ s Law –keep or flip equations. Add em up.
3. Hess’s law #2– standard heats of formation.
4. Bond energies – more to come on that as well.
Enthalpy Δ H
Calorimetry q=mcΔt
Heats of
Formation
ΔH
Hess’s
Law
Bond
Energy
Calorimetry
• Use water in a device to measure the heat given off or absorbed by a reaction or object.
• q= mc
t
• q is heat absorbed or lost by the water usually in
Joules
• m is the mass of the water in grams
• c is the specific heat of water (4.18 J/gxC)
• t is the change in temperature of the water K or C doesn’t matter.
Applying mcat
• Find q for the water, don’t worry about the sign of anything.
• If the water got warmer, the reaction was exothermic and
H will be negative.
• If the water got cooler, the reaction was endothermic and
H will be positive.
Hess ’ s Law
Reactants
Products
The change in enthalpy is the same whether the reaction takes place in one step or a series of steps.
You can lose one $10,000 bet or lose 10
$1,000 bets. Either way the end result is the same. You’re out 10 large!
Calculations via Hess ’ s Law
1.
If a reaction is reversed ,
H is also reversed.
N
2
(g) + O
2
(g)
2NO(g)
H = 180 kJ
2NO(g)
N
2
(g) + O
2
(g)
H =
180 kJ
2.
If the coefficients of a reaction are multiplied by an integer,
H is multiplied by that same integer.
6 NO(g)
3 N
2
(g) + 3 O
2
(g)
H =
540 kJ
Hess law example
• Thermite is powdered aluminum plus iron
III oxide creating iron and aluminum oxide.
• 2Al(s) + Fe
2
O
3
(s) Al
2
O
3
(s) + 2Fe(s)
• This is extremely exothermic.
• 2 Al + 3/2 O
2
Al
2
O
3
∆H=-1676 kJ/mol
• 2 Fe +3/2 O
2
Fe
2
O
3
∆H=-826 kJ/mol
• Which one gets flipped?
H using heats of formation
Standard heat of enthalpy for any element is zero.
Can be calculated from enthalpies of formation of reactants and products .
H rxn
° =
H f
( products )
H f
( reactants )
H using Bond Energies
Can be calculated from bond energies of reactants and products .
H rxn
=
–
( bond energies of reactants
( bond energies of products )
)
Notice this is opposite of the standard heats of formation.
In other words breaking all the bonds takes energy
(+
H) and building new molecules with new bonds releases energy (-
H).
1. C
2
H
4
(g) 2 CO
2
(g) + 2 H
For the reaction of ethylene represented above,
H rxn
(g) + 3 O
2 is -1323 kJ.
2
O(g)
What is the value of
H if the combustion produced liquid water H
2
O(l), rather than water vapor H
2
O(g)?
(
H for the phase change H
2
O(g) ⇆ H
2
O(l) is -44 kJ mol -1 .)
A) -1235 KJ
B) -1279 kJ
C) -1323 kJ
D) -1411 kJ
Question 1 Answer
D
Clue: Multiply one of the equations to make it work.
C
2
H
4
(g) + 3 O
2
(g) → 2 CO
2
(g) + 2 H
2
O(g)
2
O(g) → H
2
O(l)
H
H rxn
= -1323 kJ.
H rxn
= -44 kJ mol
Don’t flip anything. It works out for liquid water to be on the
-1 right.
You do need to multiply the 2 nd equation by 2 to get stuff to cancel.
C
2
2
H
4
(g) + 3 O
2
(g) → 2 CO
2
(g) + 2 H
H
2
O(g) → 2 H
2
O(l)
2
O(g)
H
H rxn rxn
= -1323 kJ.
= -44 kJ mol -1 x 2
= -88 kJ mol -1
Then add the two numbers together.
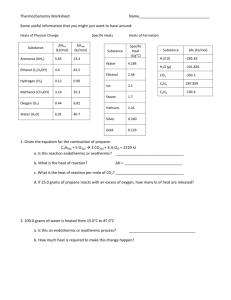
2. What is the standard enthalpy change
H ° , for the reaction:
3C
2
H
2
H °
H ° f f
(g) of C
2 of C
6
H
2
H
C
6
6
H
6
(g)
(g) is 230 kJmol
(g) is 83 kJmol -1
-1
A) -607 kJ
B) -147 kJ
C) -19 kJ
D) +19 kJ
Question 2 Answer
A
Clue: Products minus reactants
Summation = add them together
• 3C
2
H
2
(g) C
6
H
6
(g)
•
H ° f
•
H ° f of C of C
2
6
H
H
2
6
(g) is 230 kJmol
(g) is 83 kJmol -1
-1
• C
6
H
6
– (3 x C
2
H
2
) = answer
• 83 kJ – (3 x 230 kJ) =
• 83 kJ – 690 kJ = -607 kJ
3. True for the evaporation of water at 1 atm and 25 ͦC
.
A)
H>0
B)
H<0
C)
H=0
D)
H is temperature dependent
Question 3 Answer
A
Clue: sweat
When water evaporate it absorbs the energy and uses it to speed up the molecules so they can break free into a gas. Absorbing energy is +
H.
4. A 10 g sample of a metal was heated to
100 ° C and then quickly transferred to an insulated container holding 100 g of water at
20 ° C. The temperature of the water rose to reach a final temperature of 50 ° C. Calculate the heat absorbed by the water. Specific heat of water is 4 J/(gx ° C)
A) 400 kJ
B) 12 kJ
C) 8 kJ
D) 1.2 kJ
Question 4 Answer
B
Clue: You don’t always use every number given.
q = m c
t for the water since that is what the question asks.
q = 100 g x 4 J/(gx ⁰ C) x (50-20 ⁰C) q = 12000 J or 12 kJ
5. The dissolution of an ionic solute in a polar solvent can be imagined as occurring in three steps, as shown in the figure above. In step 1, the separation between ions in the solute is greatly increased, just as will occur when the solute dissolves in the polar solvent. In step
2, the polar solvent is expanded to make spaces that the ions will occupy. In the last step, the ions are inserted into the spaces in the polar solvent. Which of the following best describes the enthalpy change,
H, for each step?
A) Step 1 and Step 3 are exothermic and Step 2 takes no energy so is neither endothermic nor exothermic.
B) Step 1 and 2 add together to be 2 X Step 3.
C) Steps 2 and 3 add together to be Step 1
D) Step 3 releases energy while Step 1 and 2 absorb energy
Question 5 Answer
D
Clue: Read carefully.
Anytime you dissolve anything it takes energy to pull apart the solvent molecules and pull apart the solute particles (step 1 and 2)
When you combine them together energy is released. Thus D is the only statement that makes sense.
The steps do not have to add up to each other.
If there is more energy released than absorbed the temperature of the solution would increase.
If more energy is absorbed than released the temperature of the solution would decrease.
But the question says nothing about the temperature so we don ’ t care.
Process
Na(s) Na(g)
Na(g) Na + (g) + e -
Br
2
(g) 2 Br(g)
Br (g) + e Br (g)
H (kJ/mol m n p rxn q
Na + (g) + Br (g) NaBr(s)
Na(s) + ½ Br
2
(l) NaBr(s)
H =-361 kJ/mol r rxn
The elements Na and Br react directly to form the
) compound NaBr according to the equation above. Refer to the information above and the table below to answer the questions 6-8.
6. How much heat is released or absorbed when 0.050 mol of Br2(g) is formed from NaBr(s)?
A) 72.2 kJ is released C) 36.1 kJ is absorbed
B) 36.1 kJ is released D) 72.2 kJ is absorbed
Question 6 Answer
C
Clue: Stoichiometry
0.050 mol Br2 x 361 kJ =36.1 kJ
0.5 mole Br2
Realize the equation must be reversed to produce Br
2 so the sign of
H must be flipped.
Since the reaction has a positive
H, then heat is absorbed so 36.1 kJ is absorbed.
7. Which of the values of
H ° for a process in the table is (are) less than zero (i.e., indicate(s) an exothermic process) ?
A) r only C) p, q, and r only
B) q and r only D) n, p, q, and r
Question 7 Answer
A
Clue: Which one of these is NOT like the others.
Knocking off an electron from a neutral atom or squishing another electron on a neutral atom takes energy to make it happen. ENDOthermic
Turning a liquid into a gas or splitting apart a diatomic element take energy to make it happen. ENDOthermic .
8. Br
2
(g) + 2e 2Br (g)
Which of the following expressions is equivalent to
H ° for the reaction represented above?
A) p + q
B) p - q
C) p + 2q
D) (p/2)- q
Question 8 Answer
C
Clue: Hess’s law
Value p is all set to match the target equation.
Value q must be multiplied x 2 to match.
Nothing needs to be flipped. Just add.
9. 4 NH
3
(g) + 3O
2
(g) 2 N
2
(g) + 6 H
2
O(g)
If the standard molar heats of formation of ammonia, NH
3
H
2
(g), and gaseous water,
O(g), are -46 kJ/mol and -242 kJ/mol, respectively, what is the value of
H ° for the reaction represented above?
298
A) -190 kJ/mol rxn
B) -290 kJ/mol rxn
C) -580 kJ/mol rxn
D) -1,270 kJ/mol rxn
Question 9 Answer
D
Clue: What is the heat of formation for an element?
Heats of formation products
-Heats of formation of reactants
(6 x -242 kJ) – (4x -46 kJ) =
-1268 kJ
The nitrogen and oxygen have heats of formation of zero.
10. ½ H
2
(g) + ½ I
2
(s) HI(g)
H = 26 kJ/mol rxn
½ H
2
(g) + ½ I
2
(g)-> HI(g)
H = -5.0 kJ/mol rxn
A)
B)
C)
D)
Based on the information above, what is the enthalpy change for the sublimation of iodine, represented below?
I
2
(s) I
2
(g)
62 kJ/mol rxn
21 kJ/mol rxn
31 kJ/mol rxn
42 kJ/mol rxn
Question 10 Answer
A
Clue: flip and multiply
½ H
2
(g) + ½ I
2
½ H
2
(g) + ½ I
2
(s) HI(g)
H = 26 kJ/mol rxn
(g) HI(g)
H = -5.0 kJ/mol rxn
Multiply each equation by 2 to get the coefficients to match.
The 2 nd equation must be flipped.
H
2
(g) + I
2
(s) 2 HI(g)
2 HI(g) H
2
(g) + I
2
H = 52 kJ/mol rxn
(g)
H = +10. kJ/mol rxn
Short Free Response 1
(3 points possible)
Free Response 2 (9 pts possible)