biochem 18 [3-12
advertisement

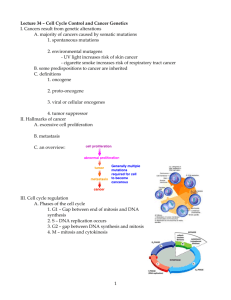
Chapter 18 Molecular Biology of Cancer 1. What is the Philadelphia chromosome? What does it cause? t(9;22) the resultant Bcr-Abl fusion protein causes CML 2. How does UVB damage cells? How is this repaired? Forms pyrimidine dimers in DNA Repaired by nucleotide excision repair pathways, which can be overwhelmed 3. How do nitrates cause cancer? Nitrates methylate guanine nucleotides 4. What DNA abnormality causes Burkitt lymphoma? c-myc gene disruptions, usually t(8;14) 5. What are proto-oncogenes? How do oncogenic viruses cause cancer? Proto-oncogenes are normal cell cycle genes that can cause cancer, usually when they suffer gain of function mutations Oncogenes from viruses may integrate into host genome, or strong promoters may be inserted 6. What do brca genes do? Besides breast cancer, what cancers is brca1 linked to? brca2? How does human epidermal growth-factor receptor 2 (HER2) influence breast cancer? Tumor-supressors Ovarian cancer Pancreatic cancer and male breast cancer HER2 is also overexpressed in 10-20% of breast cancer cases and signals a poor prognosis i. Herceptin is an antibody drug directed towards HER2 7. What kind of mutation in oncogenes leads to cancer? What kind of mutation occurs in tumorsuppressor genes and lead to cancer? Gain of function + oncogene = cancer Loss of function + suppressor = cancer 8. How does Ras beome oncogenic? Outline biochemical steps following Ras activation: Ras is converted to oncogenic form by a point mutation that decreases its GTPase activity Ras=> Raf => MEK=> MAPK => myc, fos, AP-1; AP-1=> jun and fos 9. How is the cell cycle regulated? Outline the regulation pathway. What happens with E2F? cdk4/cdk6 + Cyclin D=> Cyclin D-cdk4/cdk6 complex => phosphorylates Rb => E2F is freed to increase gene transcription and progress the cell cycle E2F induces cyclin E and cyclin A; Cyclin E-cdk2 hyperphosphorylates Rb; Cyclin A-cdk2 inactivates E2F 10. What is the function of CKIs in the cell cycle? What are the two families of CKI? CKIs inhibit cyclinD-cdk4/6 complexes Kip/Cip family (p21, p27, p57): broad specificity INK4 family (p15-p19): specific for cyclin D-cdk4/cdk6 11. Through what intermediaries does p53 stop the cell cycle and repair DNA (2)? How does p53 induce apoptosis, and why? What does inheritance of a p53 mutation lead to? Activates p21 and DNA repair enzyme GADD45 If DNA repair is not successful, p53 induces apoptosis genes bax and IGF-BP3 Li-Fraumeni syndrome [the FrauLein keeps things ordered] 12. What is the function of NF-1? NF-1 activates the GTPase domain of Raf i. Mutations cause neurofibromatosis 13. What cancer do mutations in smoothened and patched cause? Increase incidence of basal cell carcinoma 14. What do cadherin mutations do? What mutation interacts with cadherin mutations? Predispose to diffuse-type gastric cancer APC is a tumor supressor, mutations of which can lead to familial adenomatous polyposis, a form of hereditary colon cancer 15. What are caspases? What are the two classes of caspase, and what is their function? Cysteine proteases that cleave peptide bonds next to aspartate AAs Initiator caspases signal execution caspases to start apoptosis, via the death receptor pathway and the mitochondrial integrity pathway i. Initiator caspases have high numbers, 8 or above 16. Outline the death receptor pathway to apoptosis: Fas/CD95, TNF0R1, and DR3 trimerize with TNF-R1=> activate caspases 8/10 (initiators)=> activate caspases 3/6/7 (executioners); caspase 3=> Bid=> mitochondrial death 17. What molecule is essential for the mitochondrial integrity pathway? Outline the whole integrated pathway, including death receptor signals, starting with the Bcl family: Cytochrome c is released by injury and initiates the mitochondrial integrity pathway Bad, Bid, Bim => Bax, Bok, Bak => cytochrome c + Apaf=> apoptosome=> caspase 9 => caspases 3/6/7 18. How are anti-apoptotic Bcl proteins named? What is their function? Pro-apoptotics? Bcl-2, Bcl-x, and Bcl-w prevent apoptosis Prevent channel insertion and cytochrome c release from mitochondria Pro-apoptotics are any three-letter protein beginning with a B (Bax, Bak, Bok, Bad, Bin, Bim) i. Dimerize with each other 19. Why is Bcl a problem in cancers when it’s overexpressed (2)? Aside from being anti-apoptotic, Bcl also functions as a multidrug-resistance protein, meaning that it transports chemotherapeutics out of the cell 20. What drug can bind to the fusion protein Bcr-Abl? What disease is it used for? Imatinib blocks Bcr-Abl function (usually, depends on mutations) CML 21. What mutations are associated with malignant melanoma? Ras (gain of function), Cdk4 (gain of function), p53, p16, and cadherin/B-catenin