Iodination of Acetone - SaxonyLutheranLoveChemistry
advertisement

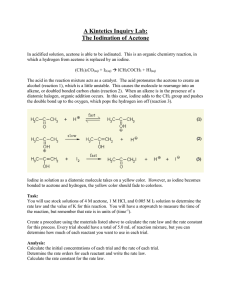
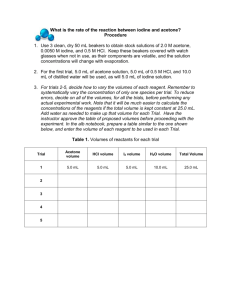
Iodination of Acetone Introduction and Goal In this experiment we will be analyzing the rate of reaction with respect to Acetone and Iodine with a Hydrogen Ion catalyst. When the color disappears (which comes from the Iodine), the reaction will be complete. Using a larger concentration of Acetone and Hydrogen Ion will ensure that Iodine is the limiting reagent (so the color will disappear). H+ (aq) + CH3COCH3 (aq) + I2 CH3COCH2I (aq) + H+ (aq) + I- (aq) Materials Needed 4.0 M Acetone Test Tubes 1.0 M HCl 10 mL Graduated cylinders 0.0050 M Iodine Solution (Lugol’s) Stop Watches Procedure We will perform multiple trials for each run. Run 1 2 3 4 4M Acetone 2 mL 4 mL 2 mL 2 mL 1M HCl 2 mL 2 mL 4 mL 2 mL Water 4 mL 2 mL 2 mL 2 mL 0.005 M I2 2 mL 2 mL 2 mL 4 mL 1. Gather 3 test tubes and a beaker 2. Add the appropriate volume of reactants to each test tube. Add the acetone, then water, then acid, and iodine last. As soon as the iodine is added, start the stop watch. 3. When the solution turns clear, the reaction is over. Record the time. 4. Repeat steps 2 and 3 for runs 2 – 4, making sure to clean out the test tubes thoroughly (and dry them) in-between each run. The data table can be found on the back… Data Table Run Elapsed Time (seconds) Average Time ∆𝑡(seconds) ∆[I₂] (M) Rate (M/sec) ∆[𝐼 2 ] ∆𝑡 1 .001M 2 .001M 3 .001M 4 .002M Rate Order Table Assuming you placed the proper volume in each trial, this is the concentrations you should have Run # [I2] (M) [Acetone] (M) [H+] (M) 1 2 3 4 0.001 0.001 0.001 0.002 0.8 1.6 0.8 0.8 0.2 0.2 0.4 0.2 Rate (from above) Analysis Determine the rate order for acetone and hydrogen ion. Then, determine the k value for the reaction. Finally, write the rate law for the reaction, including proper exponents.