Oxidation State and Electrochemistry
advertisement

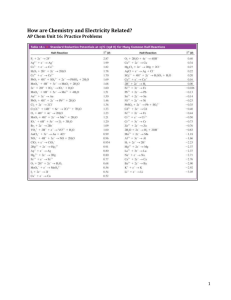
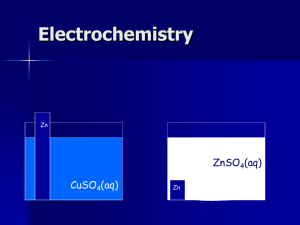
Oxidation and Reduction, Electrochemistry Charles H. Mahler Pennsylvania Multi-Region STEM Grant Immaculata University July-August 2013 Oxidation State (number) Rules 1) Pure elements ox. states = zero 2) Simple ions ox. states = charge 3) H in compounds is usually +1 4) O in compounds is usually -2 5) Sum of all ox. states in molecules is zero, in complex ions equals ion charge 6) Can use Periodic Table for other simple ox. states (Na +1, F -1, Mg +2) Daniell Cell: 1.10 volts Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) http://commons.wikimedia.org/wiki/File:Galvanic_cell_labeled.svg If A and B are opened together, water will flow from B to A. If A and C are opened together, water will flow from C to A. If D and E are opened together, water will flow from E to D. If F and B are opened together, water will flow from F to B. Instead of figuring out their exact heights, or listing all possible combinations, to predict the direction of flow all we need is to list these tanks by height: (Highest) E > F > B > C > A > D (Lowest) Figure taken from and examples adapted from http://bouman.chem.georgetown.edu/S02/lect25/lect25.htm Standard Reduction Potentials All written as reductions, listed in order from highest voltage to lowest Table taken from http://bouman.chem.georgetown.edu/S02/lect25/lect25.htm To make a cell, you need both an oxidation and a reduction The standard potential (voltage) for a galvanic cell is just: Eocell = Eoreduction- Eooxidation Eocell = Eoreduction- Eooxidation Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) (aq) + Cu (s) 0.34 V Zn2+ (aq) + 2e- Zn (s) –0.76 V 2+ Cu 2e Eocell = 0.34 V – (–0.76 V) = 1.10 V Electrochemistry Vocabulary I Galvanic cell – has a positive potential (voltage) and is a spontaneous reaction, used to do electrical work / is a source of energy (an example is a battery) Anode – electrode where oxidation takes place in a galvanic cell Cathode - electrode where reduction takes place in a galvanic cell Electrochemistry Vocabulary II Electrolytic cell - has a negative potential (voltage) and is NOT a spontaneous reaction, consumes electrical work / needs an outside source of energy to run (an example is a battery being charged, or splitting water into hydrogen and oxygen) Galvanized – a metal (usually iron or steel) that has a layer of zinc (Zn) applied to prevent rusting has been galvanized. The zinc will oxidize before the iron will, protecting the iron. Rusting vs. Galvanized metal Left image: http://commons.wikimedia.org/wiki/File:Rust_1.jpeg Right image: http://commons.wikimedia.org/wiki/File:Feuerverzinkte_Oberfl%C3%A4che.jpg