Chem. 31 * 9/15 Lecture
advertisement

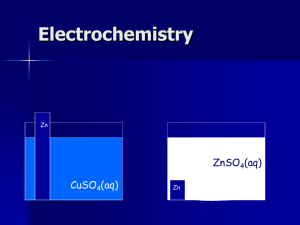
Chem. 133 – 2/25 Lecture Announcements I • Exam 1 – is scheduled for Tuesday – will cover Electronics plus some of electrochemistry (up to but not including the Nernst Equation) – will review topics after covering the parts of electrochemistry on Exam 1 – no help session – but I can have an additional office hour Friday 9 to 10 • Homework Set 1.3 – – – – Due Date is 3/1 (Tuesday for exam) Nothing collected Shortened (last problem is now Harris 13.10) Solutions have been posted Announcements II • Lab – Finishing Set 2 Period 1 labs today (still make up day if needed) – On Tuesday we start the Set 2 Period 2 labs – I will Add to the table (for Period 3 and Period 4 labs) • Today’s Lecture – Electrochemistry Topics on Exam 1 • example: charge of battery problem • galvanic cells • electrolytic cells – Review of material on Exam 1 – Electrochemistry Topics not on Exam I (if time) • the Nernst equation and its applications Electrochemistry Fundamental Equations • Example problem: A NiCad battery contains 12.0 g of Cd that is oxidized to Cd(OH)2. How long should the battery last if a motor is drawing 421 mA? Assume 100% efficiency. Electrochemistry Galvanic Cells • What are galvanic cells? GALVANIC CELL – Cells that use chemical reactions to generate electrical energy – Batteries are examples of Zn(s) useful galvanic cells – Example reaction voltmeter Ag(s) Zn(s) + 2Ag+ → Zn2+ + 2Ag(s) – If reactants are placed in a beaker, only products + heat are produced – When half reactions are isolated on electrodes, ZnSO4(aq) electrical work can be produced AgNO3(aq) Salt Bridge Electrochemistry Galvanic Cells • Description of how example cell works GALVANIC CELL – Reaction on anode = oxidation voltmeter – Anode = Zn electrode (as the Eº for Zn2+ is less than for that for Ag+) Zn(s) – So, reaction on cathode must be reduction and involve Ag – – Oxidation produces e , so anode has (–) charge (galvanic cells only); current runs from cathode to anode – Salt bridge allows replenishment of ions as cations migrate to cathode and anions toward anodes ZnSO4(aq) Zn(s) → Zn2+ + 2e- Ag+ + e- → Ag(s) + Salt Bridge Ag(s) AgNO3(aq) Electrochemistry Galvanic Cells • Cell notation GALVANIC CELL – Example Cell: voltmeter Zn(s)|ZnSO4(aq)||AgNO3(aq)|Ag(s) Zn(s) Ag(s) “|” means phase boundary left side for anode (right side for “||” means salt bridge cathode) AgNO3(aq) ZnSO4(aq) Salt Bridge Electrochemistry Galvanic Cells • Given the following cell, write the cell notation: GALVANIC CELL voltmeter – reads +0.43 V Pt(s) + – Ag(s) AgCl(s) FeSO4 (aq), Fe2(SO4)3(aq) NaCl(aq) Salt Bridge Electrochemistry Galvanic Cells • Example Questions – Given the following cell, answer the following question: MnO2(s)|Mn2+(aq)||Cr3+(aq)|Cr(s) – What compound is used for the anode? – What compound is used for the cathode? – Write out both half-cell reactions and a net reaction Electrochemistry Standard Reduction Potential • A half cell or electrode, is half of a galvanic cell • A standard electrode is one under standard conditions (e.g. 1 M AgNO3(aq)) Pt(s) • Standard reduction potential (Eº) is cell potential when reducing electrode is coupled to standard hydrogen electrode (oxidation electrode) • Large + Eº means easily reduced compounds on electrode H2(g) • Large – Eº means easily oxidized H+(aq) compounds on anode Ag(s) AgNO3(aq) Electrochemistry Electrolytic Cells • Used in more advanced electrochemical analysis (not covered in detail) • Uses voltage to drive (unfavorable) chemical reactions • Example: use of voltage to oxidize phenol in an HPLC electrochemical detector (E° of 0 to 0.5 V needed) anode (note: oxidation driven by voltage, but now + charge) cathode (reduction, - charge) Exam 1 Topics to Know A. B. Instrument Performance Measures know main measures discussed in class (e.g. sensitivity, selectivity, accuracy, etc.) Electronics 1. 2. 3. 4. DC circuits (know and be able to apply: Kirchhoff’s Laws, Ohm’s Law, Power Law) AC Circuits and Fourier Transforms (be able to determine frequency, have qualitative understanding of Fourier Transformation of time dependence to frequency dependence) RC Circuits (know quantitatively for for step changes in voltage, know qualitative effects for other changes such as noise) Analog to Digital Signal Conversion (know how to convert between decimal and binary, and between digital signal and voltage, be able to estimate uncertainty in digitizer, significance of input range, know A/D performance parameters) Exam 1 Topics to Know – cont. B. Electronics – cont. 5. Measurements with digital voltmeters (know how these can be used for current and resistance measurements; know errors associated with measurements) 6. Transducers (know how a few of each type work + how signal is measured) 7. Operational Amplifiers (know general uses) 8. Noise (know how to calculate S/N and limit of detection; know main types of noise; know how to calculate thermal or shot noise; know effect of signal averaging; know ways to reduce noise) Exam 1 Topics to Know – cont. C. Electrochemistry (Ch. 13) 1. Redox reaction knowledge (be able to identify elements being oxidized and reduced, be able to balance half and full reactions). 2. Be able to relate charge to moles of redox reactants consumed and to current and time. 3. Know how to calculate electrical energy and relate it to chemical energy. 4. Be able to identify anodes/cathodes in galvanic or electrolytic cells and their charge. 5. Know what the standard potential is a measure of. Exam 1 Equations Given On Exam 1. 2. 3. 4. 5. VR in response to step change in DVin in RC circuit (VR = DVin·e-t/RC Equation for standard deviation Equation for converting between voltage to A/D board and decimal number recorded (decimal # = (V – Vmin)2n/(Vmax – Vmin) Equations for thermal and shot noise Definition of minimum observable signal (e.g. 3s for use in calculating limit of detection) You are responsible for all other equations (e.g. V = IR, P = IV, etc.) Constants will also be provided Electrochemistry The Nernst Equation • The Nernst Equation relates thermodynamic quantities to electrical quantities for a cell reaction • Thermodynamics: – ΔG = ΔGº + RTlnQ ΔG = free energy, Q = reaction quotient – so, -nFE = -nFEº + RTlnQ, or E = Eº – (RT/nF)lnQ – more often seen as: E = Eº – (0.05916/n)logQ (although only valid at T = 298K) – Note: in calculations, E is for reductions (even if oxidation actually occurs at that electrode) – Equation for electrodes or full cells, although text uses Ecell = E+ – E- where + and – refer to voltmeter leads – Best to use activities in Q (even though we will just use concentrations) Electrochemistry The Nernst Equation • Example: Determine the voltage for a Ag/AgCl electrode when [Cl-] = 0.010 M if Eº = 0.222 V (at T = 25°C)? Electrochemistry Applications of The Nernst Equation • Examples: – The following electrode, Cd(s)|CdC2O4(s)|C2O42- is used to determine [C2O42-]. It is paired with a reference electrode that has an E value of 0.197 V (vs. the S.H.E.) with the reference electrode connected to the + end of the voltmeter. If Eº for the above reduction reaction is -0.522 V, and the measured voltage is 0.647 V, what is [C2O42-]?