Teacher Notes - OISE-IS-Chemistry-2011-2012
advertisement

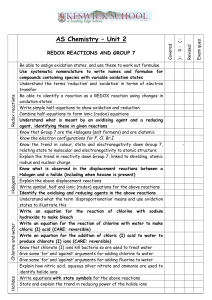
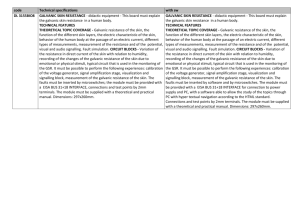
The Galvanic Cell Laboratory: Teacher Notes Big Ideas Oxidation and reduction are paired chemical reactions in which electrons are transferred from one substance to another in a predictable way. The control and applications of oxidation and reduction reactions have significant implications for industry, health and safety, and the environment. Overall Expectations A1. Demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and planning, performing and recording, analyzing and interpreting, and communicating). F2. Investigate oxidation-reduction reactions using a galvanic cell, and analyze electrochemical reactions in qualitative and quantitative terms. F3. Demonstrate an understanding of the principles of oxidation-reduction reactions and the many practical applications of electrochemistry. Specific Expectations A1.2 Select appropriate instruments (e.g., glassware, calorimeter, thermometer) and materials (e.g., chemical compounds and solutions), and identify appropriate methods, techniques, and procedures, for each inquiry. A1.4 Apply knowledge and understanding of safe laboratory practices and procedures when planning investigations by correctly interpreting Workplace Hazardous Materials Information System (WHMIS) symbols; by using appropriate techniques for handling and storing laboratory equipment and materials and disposing of laboratory materials; and by using appropriate personal protection (e.g., wearing safety goggles). A1.5 Conduct inquiries, controlling relevant variables, adapting or extending procedures as required, and using appropriate materials and equipment safely, accurately, and effectively, to collect observations and data. A1.6 Compile accurate data from laboratory and other sources, and organize and record the data, using appropriate formats, including tables, flow charts, graphs, and/or diagrams. A1.9 Analyze the information gathered from research sources for logic, accuracy, reliability, adequacy, and bias. A1.10 Draw conclusions based on inquiry results and research findings, and justify their conclusions with reference to scientific knowledge. A1.11 Communicate ideas, plans, procedures, results, and conclusions orally, in writing, and/or in electronic presentations, using appropriate language and a variety of formats (e.g., data tables, laboratory reports, presentations, debates, simulations, models). A1.12 Use appropriate numeric, symbolic, and graphic modes of representation, and appropriate units of measurement (e.g., SI units, imperial units). A1.13 Express the results of any calculations involving data accurately and precisely, to the appropriate number of decimal places and significant figures. F2.1 Use appropriate terminology related to electrochemistry, including, but not limited to: halfreaction, electrochemical cell, reducing agent, oxidizing agent, redox reaction, and oxidation number [C]. F2.2 Conduct an inquiry to analyze, in qualitative terms, an oxidation-reduction (redox) reaction [PR, AI, C]. F2.3 Write balanced chemical equations for oxidation-reduction reactions, using various methods including oxidation numbers of atoms and the half-reaction method of balancing [AI, C]. F2.4 Build a galvanic cell and measure its cell potential [PR, AI]. F2.5 Analyze the processes in galvanic cells, and draw labeled diagrams of these cells to show the oxidation or reduction reaction that occurs in each of the half-cells, the direction of electron flow, the electrode polarity (anode and cathode), the cell potential, and the direction of ion movement [AI, C]. F3.2 Identify the components of a galvanic cell, and explain how each component functions in a redox reaction. F3.3 Describe galvanic cells in terms of oxidation and reduction half-cells whose voltages can be used to determine overall cell potential. Rationale The Galvanic cell is the heart of the electrochemistry unit building on prior knowledge surrounding redox reactions as well as being tied into the STSE component of the unit. As the Galvanic cell is an enduring learning concept, it is fitting that it be one of the major labs being conducted during the electrochemistry unit. Prior Knowledge Both the teacher and the student should be familiar with the following concepts before conducting this laboratory: Oxidation and Reduction Reactions: which gains/loses electrons. Oxidation Numbers. Balancing Redox Equations: Oxidation Number method, Half-reaction method. Predicting Redox Reactions: Oxidizing Agent/Reducing agent. Basic circuitry knowledge: SNC1D. Components of an Electric cell. Basic Galvanic cell knowledge: cell notation, setup and components. Laboratory Set-up When the students come in to the classroom, their pre-lab will be checked before any equipment is given out (AfL). The students will work in pairs (or groups of 3 if there is not enough equipment) and each group will come up (3 lines) and collect the materials required and bring them back to their lab benches. Each group would label their beakers. Each group will then come back to the front and pour the corresponding chemicals to the beakers that they labeled and bring them to their lab bench. Students will conduct the lab and clean up when there is only ten minutes of class remaining. Students will then hand in their worksheet to be marked (AoL). v Legend This lab setup refers to Rm 322 UTschools. Please check the room that you will be working in to modify setup. Exit. Materials Pickup stations. Chairs, Coats, Books. Lab benches Students (Groups of Three). Inorganic waste materials disposal. Safety Checklist for teachers to ensure a safe laboratory. MSDS sheets are readily available for students. Prelab is checked to ensure completion. Goggles, lab apron (optional) and gloves (optional) are supplied. - NOTE: Students should test to see if they are allergic to gloves. All chemical waste goes into the inorganic waster beaker located at the back of the room (refer to diagram). Waste materials are put into the garbage. Equipment is cleaned and put back in their proper place. Protocol List Prepare the following solutions: 0.1M Pb(NO3)2 – 300 ml. 0.1M Cu(NO3)2 – 300 ml. 0.1M Zn(NO3)2 – 300 ml. 0.1M FeCl3 – 300 ml. 0.2M KNO3 – 850 ml. Equipment Amounts 10 voltmeters with accompanying alligator clips. 50 rectangular strips of filter paper. 45x 50ml beakers. 10x 250ml beakers. 10x Iron nail, copper strip, lead strip, and zinc strip. Ensure all glassware is cleaned and voltmeters are tested and work. ALL waste must be disposed of in the “Inorganic waste beaker” found in the fume hood. Collect class amount of Goggles. Masking tape and markers for labelling. Prelab checked for completion. 30x Worksheets photocopied.