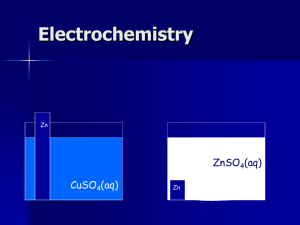
Chem. 31 * 9/15 Lecture
advertisement

Chem. 133 – 2/23 Lecture Announcements I • Homework Set 1.2 – I posted solutions on Friday, but then found a few errors (on 1.2.2 and 1.2.5) when grading the problems. These now have been corrected. • Return Q2 and HW1.2 • Exam 1 – is scheduled for next Tuesday – will cover Electronics plus some of electrochemistry (what I’m planning on covering today) – format: part multiple choice/short answer, part problems – see S’15 class website for example exam (link now working on class website) – will review topics on Thursday – Can have a review session Monday 11 to 12? or 12 to 1? Announcements II • Electronics Lab Reports – due today • Term Project Topics due (again, no penalty for being late, I can give a brief description of possible STORC projects for those interested) • Today’s Lecture – Noise – just questions not gotten to last time – Electrochemistry • • • • • intro redox balancing definitions galvanic cells electrolytic cells Noise Questions 1. 2. 3. 4. 5. 6. 7. What type of noise is likely to be present when using thermocouples to measure temperature? Why is modulation normally required to reduce 1/f noise? What is the percent noise on a current producing transducer which generates signal over a 1000 Hz band if the signal is 10 nA? if the signal is 2.0 pA? What specific type of noise is reduced best by shielding electronics? How would use of a low pass filter reduce shot noise? Suggest one method for reducing thermal noise. What type of noise is not effectively reduced by using a low pass filter? Electronics Additional Questions 250 200 a) Voltage (mV) • Answer the questions 1-3 from the following plots which were obtained from background measurements (instrument noise): 150 100 50 0 1. Which plot is most likely shows 1/f noise: ______________________ -50 0 50 100 150 Time (ms) 180 160 will produce a plot with a peak at 55 Hz: ______________ 140 120 Voltage (mV) 2. Which plot when Fourier transformed b) 100 80 60 40 20 0 0 40 60 80 100 120 140 160 time (ms) 120 100 Voltage (mV) 3. If plot c) shows noise from a GC signal in which peaks typically are on the c) order of 2 s (2000 ms) wide, what can be done to reduce the noise? 20 80 60 40 20 0 0 50 100 Time (ms) 150 Electrochemistry Overview • Applications – quantitative analysis • potential measurement methods (e.g. pH electrode) • current based measurements (amperometry) – qualitative analysis (voltammetry) – note: potential normally gives qualitative information and current quantitative measurements – HPLC/IC detectors • Why Use? – lower cost (both for instruments and supplies) – high sensitivity possible (particularly mass sensitivity) – simpler equipment, more useful for field, in-situ type measurements Electrochemistry Redox Reactions • Reduction = loss of charge – e.g. Fe3+ + e- → Fe2+ • Oxidation = gain in charge – e.g. Pb2+ + 2H2O → PbO2(s) + 4H+ + 2e- (Pb goes from +2 to +4) • Balancing reactions – review steps in general chemistry book – example: Zn(s) + Cr2O72- → Zn2+ + Cr3+ – note: based on methods used for problems in this book, full cell balancing may not be needed Electrochemistry Fundamental Equations • Relationship between charge, energy and current – redox reactions involve the exchange of electrons – when the exchange occurs on an electrode surface, current can be measured – Total charge transfer = q = nF, where • n = moles of electrons in reaction and • F = Faraday’s constant = 96500 C/moles e• F = NAvogadro·e (e = elementary charge = 1.6 x 10-19 C) – Current Produced = I = dq/dt • or q = ∫I·dt (or = I·t under constant current conditions) • can be used to determine battery lifetime Electrochemistry Fundamental Equations • Relationship between charge, energy and current (continued) – Electrical work = E·q (E = potential in volts) – and ΔG = -E·q = -nFE – under standard conditions (1 M reactant/product conc., 298K, etc.), ΔGº = -nFEº – ΔGº are given in Tables and allows calculation of K values – Eº, standard reduction potential, also given in Tables (see Appendix H), but for “half-reactions” Electrochemistry Fundamental Equations • Example problem: A NiCad battery contains 12.0 g of Cd that is oxidized to Cd(OH)2. How long should the battery last if a motor is drawing 421 mA? Assume 100% efficiency. Electrochemistry Galvanic Cells • What are galvanic cells? GALVANIC CELL – Cells that use chemical reactions to generate electrical energy – Batteries are examples of Zn(s) useful galvanic cells – Example reaction voltmeter Ag(s) Zn(s) + 2Ag+ → Zn2+ + 2Ag(s) – If reactants are placed in a beaker, only products + heat are produced – When half reactions are isolated on electrodes, ZnSO4(aq) electrical work can be produced AgNO3(aq) Salt Bridge Electrochemistry Galvanic Cells • Description of how example cell works GALVANIC CELL – Reaction on anode = oxidation voltmeter – Anode = Zn electrode (as the Eº for Zn2+ is less than for that for Ag+) Zn(s) – So, reaction on cathode must be reduction and involve Ag – – Oxidation produces e , so anode has (–) charge (galvanic cells only); current runs from cathode to anode – Salt bridge allows replenishment of ions as cations migrate to cathode and anions toward anodes ZnSO4(aq) Zn(s) → Zn2+ + 2e- Ag+ + e- → Ag(s) + Salt Bridge Ag(s) AgNO3(aq) Electrochemistry Galvanic Cells • Cell notation GALVANIC CELL – Example Cell: voltmeter Zn(s)|ZnSO4(aq)||AgNO3(aq)|Ag(s) Zn(s) Ag(s) “|” means phase boundary left side for anode (right side for “||” means salt bridge cathode) AgNO3(aq) ZnSO4(aq) Salt Bridge Electrochemistry Galvanic Cells • Given the following cell, write the cell notation: GALVANIC CELL voltmeter – reads +0.43 V Pt(s) + – Ag(s) AgCl(s) FeSO4 (aq), Fe2(SO4)3(aq) NaCl(aq) Salt Bridge Electrochemistry Galvanic Cells • Example Questions – Given the following cell, answer the following question: MnO2(s)|Mn2+(aq)||Cr3+(aq)|Cr(s) – What compound is used for the anode? – What compound is used for the cathode? – Write out both half-cell reactions and a net reaction Electrochemistry Standard Reduction Potential • A half cell or electrode, is half of a galvanic cell • A standard electrode is one under standard conditions (e.g. 1 M AgNO3(aq)) Pt(s) • Standard reduction potential (Eº) is cell potential when reducing electrode is coupled to standard hydrogen electrode (oxidation electrode) • Large + Eº means easily reduced compounds on electrode H2(g) • Large – Eº means easily oxidized H+(aq) compounds on anode Ag(s) AgNO3(aq) Electrochemistry Electrolytic Cells • Used in more advanced electrochemical analysis (not covered in detail) • Uses voltage to drive (unfavorable) chemical reactions • Example: use of voltage to oxidize phenol in an HPLC electrochemical detector (E° of 0 to 0.5 V needed) anode (note: oxidation driven by voltage, but now + charge) cathode (reduction, - charge)