Accuracy with Measurements
advertisement

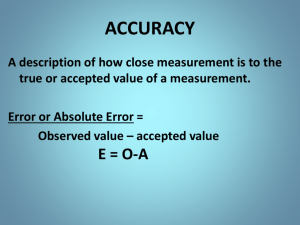
Accuracy v. Precision • Quality of Data • Precision: • Well-grouped together • Results are repeatable • Compare to other repeated measurements • Accuracy: • Close to actual value • Compare to correct value Accuracy v. Precision Precise – all the hits are close together Not accurate – the hits are not close to bulls eye Not precise – none of the hits are close together Not accurate – none of the hits are close to bulls eye Precise – all the hits are close together Accurate – all the hits are near the bulls eye 2 ways to show accuracy & precision Significant Figures Percent Error Accuracy with Measurements • Significant Figures : numbers that show precision in measurements • Key question: Is there a decimal? YES: come from left, 1st number (non-zero) and anything after it is significant 0.001 ml 4.7200 mL 82.0 sec Accuracy with Measurements • Significant Figures : numbers that show precision in measurements • Key question: Is there a decimal? NO: come from right, until you hit a number and everything past it is significant 5090 dm 7000 meters * Scientific Notation: don’t count it 6.02x1023 Accuracy with Measurements • Significant Figures • Practice: http://science.widener.edu/svb/tutorial/sigfigure s.html • Rounding to Significant Figures 314.7 314.721 (four) 8800 8792 (two) 87.1 87.073 (three) 0.001775 0.0018 (two) -5 -5 4.36x10 4.3622x10 (three) 3 1.0x10 1040 (two) Accuracy with Measurements • Practical Applications: Estimating in Measurements • Always estimate one decimal place past the actual accuracy of the measuring device’s scale Accuracy with Measurements • Practical Applications: Estimating in Measurements Accuracy with Measurements • Significant Figures in Calculations • Multiplication and Division • Answer’s sig figs = least sig figs • Example: 3.126x10-6 x 2.8x10-7 = 8.8x10-13 Accuracy with Measurements • Practice! Use your calculator to find the following answers. Report it in scientific notation. Round to the proper amount of sig figs 1) 5.231 x 10-4 ÷ 1.49 x 104 = 3.51x10-8 2) 9.62 x 105 x 5.4 x 106 = 5.2x1012 3) 2.62x10-4 ÷ 3.14x10-8 = 8344= 8.34x103 4) 7.72x10-2 x 5.74x10-2 = .004431= 4.43x10-3 -9 -5 4 3x10 5) 8.22x10 ÷ 3 x10 = Accuracy with Measurements • Significant Figures in Calculations • Addition & Subtraction • Answer’s decimal places = least decimal places • Examples: a) 128.112 g – 18.92 g = 109.19 g b) 100 g + .01 g = 100 g c) 9.27x10-4 + 9.237x10-5 = 1.019x10-3 g .000927 + .00009237 = .001019 g Accuracy with Measurements • Practice! Use your calculator to find the following answers. Report it in scientific notation. Round to the proper amount of sig figs 1) 39.64 + 1.3 = 40.9 2) 195.4 – 193 = 2 3) 5.8465x102 + 1.2x103 = 1.8x103 4) 9x102 + 5x102 = 1.4x103 5) 46.8 – 41.4 = 5.4 WebAssign Hint • Just assume that you should use sig figs from here on out. Also, on the WebAssign, you will see this: This symbol means you must put the correct number of significant figures on web assign (not always 4) Data Analysis • Percent error: shows how accurate experimental values are by determining how much error they have Actual = correct answer Experimental = what you got Data Analysis • Practice: The density of silver is 10.49 g/ml. If I measured it to be 11.1 g/ml, what is my percent error? 5.82% error Data Analysis • Practice: The density of gold is 19.32 g/cm3. What is the percent error of the following experimental measurements? a) 20.1 g/cm3 4.04 % error b) 19.51 g/cm3 0.9834 % error c) 19.7 g/cm3 1.97 % error d) 18.90 g/cm3 2.174 % error Accuracy with Measurements • Practice Problems Due tomorrow