Measurement & Data Processing
advertisement

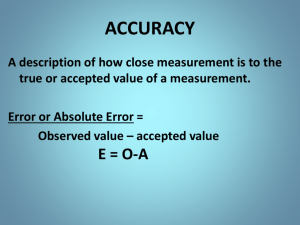
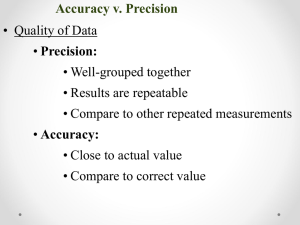
Measurement & Data Processing IB Chem • Objective: demonstrate knowledge of measurement & data processing. • Warm up: Explain the difference between accuracy and precision. Challenge… • Hemoglobin (C2952H4664O832S8Fe4) is the oxygen carrier in blood. • Calculate its molar mass. An average adult has about 5.0 liters of blood. Every milliliter of blood has approximately 5.0 x 109 erythrocytes, or red blood cells, and every red blood cell has about 2 x 108 hemoglobin molecules. • Calculate the mass of hemoglobin molecules in grams in an average adult. Significant Figures • The certain (known) digits and one estimated digit of each measurement are significant. • Remember! Every time you make a measurement, you record all of the certain digits and one estimated digit. 200.54 g Significant Figures 5508 has 4 sig figs 1. Non-zeros are always significant. Rules for Sig Figs 2. Zeros between non-zeros are significant. 3. All final zeros to the right of the decimal are significant. (estimated value) 67.80 has 4 sig figs 4. Placeholder zeros are NOT significant. – Zeros preceding significant digits. – Zeros following significant digits without a decimal point. Significant Figures Sample Problem: 17.20 4.137 + 26.6 47.937 (.01) (.001) (.1) Correct Answer: 47.9 Significant Figures Sample Problem: 14.3 1.0200 x 0.005 0.07293 (3 sig figs) (5 sig figs) (1 sig fig) Correct Answer: 0.07 Scientific Notation In chemistry, we work with very large and very small numbers. Number of particles in a mole = 602200000000000000000000 Mass of an electron = 0.000000000000000000000000000000911kg Scientific Notation 1. 2. 3. 4. Identify the significant digits Write out the significant digits as a number greater than 1 but less than 10 602200000000000000000000 There are 4 sig figs in this number 6.022 is < 1 and > 10 The decimal was moved 23 places Count the number of places you had to move the decimal to complete step 1 6.022 x 1023 Write this number of decimal places as an exponent to 10 When the decimal place is moved to the left, the exponent is positive. Things you already know Types of Uncertainty/Error • Random: • Systematic Error introduced Error introduced has an equal will always be too probability of being high or too low. too high or too low 50/50 chance Ex: Air bubbles in Ex: Door open on thermometer analytical balance Using equipment • Analogue +/- Half of the smallest division • Digital +/- The smallest scale division Uncertainty • Absolute ± half of the smallest division • Percent Absolute uncertainty divided by the measurement x 100 Always include units 35.0 ± 0.5 cm3 0.5/35.0 *100 = 1.4% Uncertainties in Calculations Addition/Subtraction Add absolute uncertainties + 23.0 ± 0.1 cm3 34.0 ± 0.5 cm3 57.0 ± 0.6 cm3 Uncertainties in Calculations Multiplication/Division 1. Multiply or divide measured numbers 3.0 ± 0.1 cm3 x 4.0 ± 0.5 cm3 12 2. Convert absolute uncertainties into percents 0.1/3.0 * 100 = 3% 0.5/4.0 * 100 = 10% (remember to use multiplication/ division sig fig rules) 3. Add percents 12 cm3 ± 13% 4. Convert back to absolute 13% = 100 * x/12.0 12 ± 1.6 cm3 Percent error A measure of how close the experimental value is to the accepted/known value Not to be confused with percent uncertainty… (accepted value – experimental value) X 100 accepted value Equipment/technique uncertainty compared to literature values • If % uncertainty > % error, the experimental value fits within the uncertainty range and is acceptable; the differences in the experimental and literature values is due to random errors • If % uncertainty < % error, the experimental value does not fit within the uncertainty range and is unacceptable; the differences in the experimental and literature values is due to systematic errors Equipment/technique uncertainty compared to literature values • Example: % uncertainty is 20 g +/- 5% < % error is 10% This indicates the data should fall between 19 and 21 grams. The error of 10% falls outside of this. Meaning the accepted or literature values are outside of this range produced. This must be due to systematic error and is UNACCEPTABLE! Accuracy vs. Precision Accuracy vs. Precision Accuracy measures how close a measured value comes to a predetermined target value (the set volume on your pipettor). accurate precise accurate not precise Reproducibility (precision) measures how close repeated values are to one another. These concepts can be visualized using these cartoon (idealized) bulls-eye diagrams. Notice that accuracy and precision can vary independently, so they can be evaluated independently, as well. Test yourself on identifying if these examples are precise, accurate, neither, or a mix not accurate precise not accurate not precise Graphing video Graphing • Always include: – Title – Axis titles with units – A best fit line – Identification of outliers – Consistent scales – no uneven jumps Always make the graph as large as possible…maximize axis usage and paper usage Extrapolation & Interpolation • Extrapolation- Extending the graph to determine an unknown value outside the range of measured values • Interpolation- Determining an unknown value within the limits of the measured values Challenge… • Hemoglobin (C2952H4664O832S8Fe4) is the oxygen carrier in blood. • Calculate its molar mass. An average adult has about 5.0 liters of blood. Every milliliter of blood has approximately 5.0 x 109 erythrocytes, or red blood cells, and every red blood cell has about 2 x 108 hemoglobin molecules. • Calculate the mass of hemoglobin molecules in grams in an average adult. • Objective: demonstrate knowledge of measurement & data processing. • Warm up: Explain the difference between accuracy and precision.