Notes - Mr. Palermo`s Flipped Chemistry Classroom
advertisement

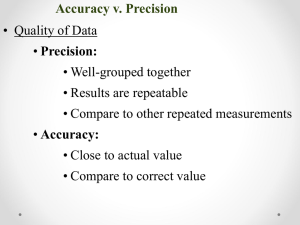
Name: Regents Chemistry: Mr. Palermo Notes: Unit 1: Math and Measurement www.mrpalermo.com 8/9/15 UNIT 1 MATH AND MEASUREMENT M R . PA L E R M O OBJECTIVE: BY THE END OF THIS VIDEO YOU WILL BE ABLE TO: LESSON 1: METRIC CONVERSIONS ü Identify base units of measurement. ü Convert between units of measurement. W W W. M R PA L E R M O . C O M WHAT IS CHEMISTRY? MATTER CAN BE DESCRIBED AS: • The study of Matter and the changes it undergoes….. • Qualitative measurements: descriptive, non-numerical observations • What is Matter? • Quantitative Measurements: are in the form of NUMBERS and UNITS. - Matter is anything that has mass and takes up space. 1 8/9/15 QUANTITATIVE MEASUREMENTS: TABLE D (BASE UNITS) • The METRIC SYSTEM (SI): System of measurement used in science and in most countries • The BASE UNITS of measurement: (Found on Reference Table D) PREFIXES: • Used to modify base units of measurement. (Found on Reference Table C) CHECK YOUR UNDERSTANDING: ü Can you identify the base unit of measurement? Example: gram (g) CONVERTING UNITS USING TABLE C WHERE ARE THE BASE UNITS? 1. Find the difference between the exponents of the two prefixes on Table C. 2. Move the decimal that many places. To the left or right? 2 8/9/15 TRICK: TURN THE TABLE ON ITS SIDE EXAMPLE 1: CONVERT 5.2 CM = ____ MM • The difference between the two factors (-­‐2 and -­‐3) is 1. • Since you are moving from a larger prefix to a smaller prefix you move the decimal one place to the right. EXAMPLE 2: CONVERT 45.5 MM = ____ M • The difference between the two factors (-­‐3 and 0) is 3. • Since you are moving from a smaller prefix to a larger prefix you move the decimal three places to the leH. CHECK YOUR UNDERSTANDING: ü Can you convert between units of measurement? EXAMPLE 3 Convert the following: 20 cm = ____________ m YOU SHOULD BE ABLE TO: ü Identify base units of measurement. ü Convert between units of measurement. 3 8/9/15 OBJECTIVE: BY THE END OF THIS VIDEO YOU WILL BE ABLE TO: ü Determine the volume of a substance ü Calculate density/mass/volume LESSON 2: DENSITY W W W. M R PA L E R M O . C O M QUANTITATIVE CALCULATIONS: • Mass: the amount of matter an object contains. (This is different than weight, which is mass plus gravity) HOW DO WE MEASURE MASS IN THE LAB? • Electronic Balance • Volume: The amount of space a substance occupies HOW CAN WE MEASURE VOLUME? • l x w x h (regular solid) - ex. V = READING A MENISCUS line 10 mL 1cm3 • Graduated cylinder (liquids) li of s ight too high proper line of sight w o lo ht to f sig ne o 10 high too ding 8 rea reading correct 6 readin g to o lo w - Read bottom of MENISCUS - ex. V = 27.5 mL graduated cylinder 4 8/9/15 MEASURING VOLUME: IRREGULAR SOLID EXAMPLE: WHAT IS THE VOLUME OF THE SOLID? Water displacement method 1. Measure initial volume 2. Measure final volume with object 3. The Difference is the volume of the object CHECK YOUR UNDERSTANDING: ü Can you Determine the volume of a substance? DENSITY • Ratio of mass of an object to its volume • Use density formula • Located on Table T EXAMPLE 1 What is the density of an object with a mass of 60 g and a volume of 2 cm3? D= D= M V CHECK YOUR UNDERSTANDING: ü Can you calculate density/mass/ volume M V 5 8/9/15 EXAMPLE 2 An object has a volume of 825 cm3 and a density of 13.6 g/cm3. Find its mass. M D = CHECK YOUR UNDERSTANDING: ü Can you calculate density/mass/ volume V HOW TO SOLVE FOR MASS OR VOLUME IF DENSITY IS NOT GIVEN: USE TABLE S Example: The volume of an aluminum sample is 251 cm3. What is the mass of the sample? CHECK YOUR UNDERSTANDING: ü Can you calculate density/mass/ volume The density of aluminum on table S is 2.70g/cm3 YOU SHOULD BE ABLE TO: • Determine the volume of a substance • Calculate density/mass/volume LESSON 3: TEMPERATURE CONVERSIONS W W W. M R PA L E R M O . C O M 6 8/9/15 OBJECTIVE: BY THE END OF THIS VIDEO YOU WILL BE ABLE TO: ü Differentiate between Kelvin and Celsius Scales ü Convert between Celsius and Kelvin temperature TEMPERATURE SCALES TEMPERATURE: • Measure of average kinetic Energy CELSIUS SCALE • Freezing point of water at 0°C. • Boiling for water at 100°C. • Below 0 is NEGATIVE. KELVIN SCALE • Water freezes at 273K and boils at 373K • Theoretical point of ABSOLUTE ZERO is when all molecular motion stops • NO NEGATIVE NUMBERS • Divisions (degrees) are the same as in Celsius 7 8/9/15 CHECK YOUR UNDERSTANDING: ü Can you differentiate between Kelvin and Celsius Scales CONVERTING BETWEEN TEMPERATURE SCALES • Formula: K = °C + 273 • Located on Table T EXAMPLE 2: What is the temperature in Celsius of an object that is 150 K? EXAMPLE 1: What is the temperature in Kelvin of an object that is 55°C ? CHECK YOUR UNDERSTANDING: ü Can you convert between Celsius and Kelvin temperature 8 8/9/15 YOU SHOULD BE ABLE TO: ü Differentiate between Kelvin and Celsius Scales ü Convert between Celsius and Kelvin temperature LESSON 4: PERCENT ERROR W W W. M R PA L E R M O . C O M OBJECTIVE: BY THE END OF THIS VIDEO YOU WILL BE ABLE TO: ACCURACY VS. PRECISION ü Differentiate between accuracy and precision ü Calculate percent error • Accuracy - how close a measurement is to the accepted or true value EXAMPLE • Precision - how close a series of measurements are to each other EXAMPLE: These students were asked to determine the density of sucrose. Sucrose has a density of 1.59 g/cm3. Which student is more accurate? Student A (g/cm3) Student B (g/cm3) Student C (g/cm3) Trial 1 1.54 1.40 1.70 Trial 2 1.60 1.68 1.69 Trial 3 1.57 1.45 1.71 Avg. 1.57 1.51 1.70 Range 0.06 0.28 0.02 9 8/9/15 CHECK YOUR UNDERSTANDING: ü Can you differentiate between accuracy and precision PERCENT ERROR • Measurement of ACCURACY - the % that the measured value is “off” from accepted value • Measured value = value you “get” • Accepted value = value you “should get” Formula is found on Table in your Reference Table: • If answer is negative, your measured value is LESS THAN the accepted value • If answer is positive, your measured value is GREATER THAN the accepted value EXAMPLE A student determines the density of a substance to be 1.40 g/mL. Find the % error if the accepted value of the density is 1.36 g/mL. CHECK YOUR UNDERSTANDING: ü Can you calculate percent error YOU SHOULD BE ABLE TO: ü Differentiate between accuracy and precision ü Calculate percent error 10 8/9/15 OBJECTIVE: BY THE END OF THIS VIDEO YOU WILL BE ABLE TO: LESSON 5: PRECISION & SIGNIFICANT FIGURES ü Identify the precision of a measuring device ü Identify the amount of significant figures in a number W W W. M R PA L E R M O . C O M SIGNIFICANT FIGURES • Indicate PRECISION of a measurement. • Recording Sig Figs - Sig figs in a measurement include the known digits plus a final estimated digit (precision of instrument) 2.38 cm EXAMPLE MEASURING LENGTH: • We know for sure that the object is more than ____, but less than ____ • We know for sure that the object is more than ____, but less than ____ • This ruler allows us to estimate the length to ______ 2 RUNNERS FINISH THE RACE IN 8 SECONDS. WHO WON? 1 2 11 8/9/15 EXAMPLE: WHAT IS THE LENGTH OF THE RED LINE? Runner 1 Runner 2 0 cm 2 3 4 5 RULES FOR COUNTING SIG FIGS CHECK YOUR UNDERSTANDING: ü Can you identify the precision of a measuring device 1 1. All non-­‐zero digits are significant. 2. Leading zeros are never significant. ex. 0.421 (3 sig figs) 3. All capTve zeros are significant. (Cap+ve is a zero between 2 other non-­‐zero digits.) ex. 4012 (4 sig figs) 4. For Trailing zeros: (zeros aHer last non-­‐zero digit) -­‐ Decimal point → significant -­‐No decimal point → not significant ex. 114.20 (5 sig figs) ex. 11,420 (4 sig figs) HOW TO COUNT SIG FIGS 1. Start counTng from LEFT to RIGHT at first NONZERO number. 2. If decimal point is present then count any trailing zeros 3. If decimal is not present don’t count trailing zeros EXAMPLE 1) 2545.300 g (7 sig figs) 2) 4530 km (3 sig figs) 3) 0.00453 m (3 sig figs) 12 8/9/15 CHECK YOUR UNDERSTANDING: ü Can you identify the amount of sig figs in a number YOU SHOULD BE ABLE TO: ü Identify the precision of a measuring device ü Identify the amount of significant figures in a number OBJECTIVE: BY THE END OF THIS VIDEO YOU WILL BE ABLE TO: ü Round answers to proper sig figs in calculations LESSON 6: ROUNDING SIG FIGS IN CALCULATIONS W W W. M R PA L E R M O . C O M WHAT DO I ROUND MY ANSWER TO? • Every measurement has some error in it. When performing calculations AN ANSWER CAN NEVER BE MORE PRECISE THAN YOUR LEAST PRECISE MEASUREMENT ROUNDING: SIG FIG IN CALCULATIONS • MulTply/Divide -­‐ Round answer to the least number of significant figures. Example: (13.91g/cm3)(23.3cm3) = 324.103g 4 SF 3 SF 3 SF 324 g 13 8/9/15 CHECK YOUR UNDERSTANDING: ü Can you round answers to proper sig figs in calculations CALCULATING SIG FIGS (CON’T) • Add/Subtract – Round to the least place value Example: 3.75 mL + 4.1 mL 7.85 mL → 7.9 mL 224 g + 130 g 354 g → 350 g YOU SHOULD BE ABLE TO: ü Round answers to proper sig figs in calculations LESSON 7: SCIENTIFIC NOTATION W W W. M R PA L E R M O . C O M OBJECTIVE: BY THE END OF THIS VIDEO YOU WILL BE ABLE TO: ü Convert numbers into scientific notation and standard notation ü Calculate mathematical operations using scientific notation SCIENTIFIC NOTATION • A way to represent large or small numbers For example: • The mass of a hydrogen atom is 0.00000000000000000000000167g. • 2 g of H2 contains 602,000,000,000,000,000,000,000 molecules. 14 8/9/15 SCIENTIFIC NOTATION IS WRITTEN AS: • The product of two numbers: a coefficient and a 10 raised to a power. • The coefficient (number written first) is always a number from 1 to 9 Example: 1.67 x 10-24 g 2 g of H2 is composed of 6.02 x 1023 molecules. CONVERTING FROM EXPANDED FORM INTO SCIENTIFIC NOTATION 1. For #’s greater than 1 move decimal to the LEFT until there’s 1 digit to its left. The number of places moved = exponent number Example: 45,450 g = CHECK YOUR UNDERSTANDING: 2. For #’s less than 1 move decimal to RIGHT stopping after the first non zero number. The number of places moved = negative exponent number ü Can you convert numbers into scientific notation and standard notation Example: 0.00453 ml = 4.53 x 10-3 ml CONVERTING FROM SCIENTIFIC NOTATION TO STANDARD NOTATION 1. Move the decimal place the number of times indicated by the exponent. 2. To the right if it is positive. 3. To the left if it is negative. CHECK YOUR UNDERSTANDING: ü Can you convert numbers into scientific notation and standard notation Example: 4.5 x 10-2 = 0.045 15 8/9/15 CALCULATING WITH SCI NOTATION USING A CALCULATOR Ex. (5.44 × 107 g) ÷ (8.10 × 104 mol) = Type on your calculator: 5.44 EXP EE 7 ÷ 8.10 EXP EE 4 EXE CHECK YOUR UNDERSTANDING: ü Can you calculate mathematical operations using scientific notation ENTER = 671.60493 = 672 g/mol = 6.72 × 102 g/mol YOU SHOULD BE ABLE TO: ü Convert numbers into scientific notation and standard notation ü Calculate mathematical operations using scientific notation 16