Ch. 1 Self Test Review

Ch. 1 Test – Self Review
Honors Chemistry
Mrs. Klingaman
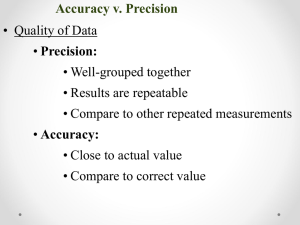
Match the statements a. Precision b. Accuracy
1. _____Separates a homogeneous solution based on differences in boiling points c. Distillation 2. _____Several measurements close to one another d. Chromatography
3. _____Separates a mixture based e. Filtration on differences in solubility
4. _____Measurements close t the true or accepted value
5. _____Separates solid & liquid mixture based on particle size
Match the statements a. Precision b. Accuracy
1. __ C __Separates a homogeneous solution based on differences in boiling points c. Distillation 2. __ A __Several measurements close to one another d. Chromatography
3. __ D __Separates a mixture based e. Filtration on differences in solubility
4. __ B __Measurements close t the true or accepted value
5. __ E __Separates solid & liquid mixture based on particle size
Measurements & Data
State whether the following data is intensive or extensive and whether it is quantitative or qualitative :
1) The chlorine gas is green in color.
2) The density of the substance is 1.33 g/mL
3) The volume of the helium gas is 23 cm 3
Measurements & Data
State whether the following data is intensive or extensive and whether it is quantitative or qualitative :
1) The chlorine gas is green in color.
intensive & qualitative
2) The density of the substance is 1.33 g/mL intensive & quantitative
3) The volume of the helium gas is 23 cm 3 extensive & quantitative
States of Matter
Describe each of the three states of matter . Be sure to include a description of if they each have definite or indefinate shapes & volumes, state a distinguishing properties for each, and give 1 elemental example of each.
States of Matter
Describe each of the three states of matter . Be sure to include a description of if they each have definite or indefinate shapes & volumes, state a distinguishing properties for each, and give 1 elemental example of each.
Solid – definite shape & volume, partciles are closely packed in ridged order & not compressible, example = iron
Liquid – indefinate shape & definite volume, particles close together & can flow, example = mercury or bromine
Gas – indefinite shape & volume, fills the volume of its container & particles very far apart, example = neon
Measurements & Data
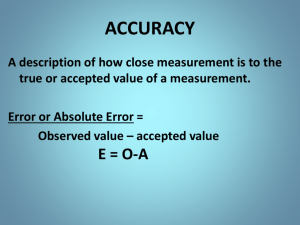
State whether the following scenarios represent high accuracy and high precision , low accuracy & high precision , or low accuracy and low precision .
1) Three volume measurements of a 4.2 mL give the following results: 2.2 mL, 3.2 mL, and 6.6 mL.
2) The density of water at room temperature is calculated to be 1.003 g/mL, 0.999 g/mL, and 1.001 g/mL.
3) The mass of a paper clip (actually 1.2 g) is found to be
0.55 g, 0.57 g, and 0.52 g.
Measurements & Data
State whether the following scenarios represent high accuracy and high precision , low accuracy & high precision , or low accuracy and low precision .
1) Three volume measurements of a 4.2 mL give the following results: 2.2 mL, 3.2 mL, and 6.6 mL.
low accuracy & low precision
1) The density of water at room temperature is calculated to be 1.003 g/mL, 0.999 g/mL, and 1.001 g/mL.
high accuracy & high precision
3) The mass of a paper clip (actually 1.2 g) is found to be
0.55 g, 0.57 g, and 0.52 g.
low accuracy & high precision
Metric Conversions:
(set them up w/dimensional analysis)
1. 100 mm = ____________ dm
2. 15.0 cm = _____________ km
3. 0.65 km = _____________ m
4. 134,076 nm = ______________ m
Temperature Conversion:
1. 15.6 ºC = ______________ K
Answers:
1. 100 mm = 1 dm
2. 15.0 cm = 1.50 x 10 -4 km
3. 0.65 km = 650 m
4. 134,076 nm = 1.34076 x 10 -4 m
Temperature Conversion:
1. 15.6 ºC = 288.6
K
Significant Figures/ Scientific Notation
Identify the # of significant figures and rewrite in scientific notation.
1. 160000
2. 160700
3. 0.000167
4. 160.0
5. 0.0160
Answers:
Identify the # of significant figures and rewrite in scientific notation.
1. 160000 (2) 1.6 x 10 5
2. 160700 (4) 1.607 x 10 5
3. 0.000167 (3) 1.67 x 10 -4
4. 160.0 (4) 1.600 x 10 2
5. 0.0160
( 3) 1.60 x 10 -2
Complete the following problems using Sig. Figs.
1. 103.36 + 12.097 + 11 =
2. 24.5 x 0.25 x 100.1 =
3. (76.2 x 15) =
4
Answers:
1. 103.36 + 12.097 + 11 = 126 ( 0 deci. places)
2. 24.5 x 0.25 x 100.1 = 610 (2 sig. figs)
3. (76.2 x 15) = 300 (1 sig. fig)
4
Use Dimensional Analysis and sig. figs to solve the following:
1. If the average student walks 3.63 miles around the school in a day, how many kilometers is this?
2. A piece of magnesium with a density of 1.74 g/cm 3 is found to have a volume of 15,600 µ L. What is the mass of this piece of Mg?
Use Dimensional Analysis and sig. figs to solve the following:
1. If the average student walks 3.63 miles around the school in a day, how many kilometers is this?
3.63 mi 5280 ft 12 in 2.54 cm 1m 1km = 5.84 km
1 mi 1 ft 1 in 100 cm 1000 m
1. A piece of magnesium with a density of 1.74 g/cm 3 is found to have a volume of 15,600 µ L. What is the mass of this piece of Mg?
1.74 g 1 cm 3 1000 mL 1 L 15,600 µ L = 27.1 g
1 cm 3 1 mL 1 L 10 6 µ L