UV-Vis & Fluorescence
advertisement

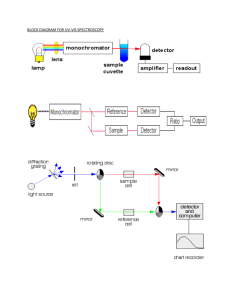
UV-Vis & Fluorescence Introduction The Thermo Evolution 600 UV-Vis (Ultraviolet-Visible spectroscopy) is an amazing instrument at its core. The instrument is able to scan a sample and determine its absorbance/reflective properties. Ultraviolet-visible light wavelengths extend from 180nm-780nm which this instrument can detect. The Shimadzu Spectrofluorometer performs a similar task, except that it looks at fluorescent properties of the sample. A light source will excite the electrons in the sample. This excitation and the returning to normal (ground state) is what this instrument detects and reports back as intensity. Purpose The purpose of this lab is to better understand the uses/how to use the UV-Vis Spectrophotometer and Spectrofluorometer. We will do this by creating calibration curves with various dilutions of a salicylic acid solution and an aspirin sample for the UV-Vis. For the Spectrofluorometer we will use quinine dissolved in 0.1M sulfuric acid solution and a tonic water sample. Procedure UV-Vis: Day One -Create 1000ppm stock solution of Salicylic Acid (SA) in hexanes -Dilute stock to an appropriate range (10, 20, 40, 60, 80ppm) -Three trials per standard -Create titration curve from data Fluorescence: Day One -Create 100ppm stock solution from 1000ppm stock solution -Dilute new 100ppm stock solution to standards (10, 5, 1, 0.1ppm) -Run on instrument UV-Vis: Day Two -Make new calibration curve -Make and run aspirin sample -One aspirin tablet crushed in mortar and pestle, dissolved in hexanes, and filtered out any remaining solid parts of tablet. -Adjust range on instrument as needed Fluorescence: Day Two -Make new calibration curve -Test Unknown -5% Tonic water and 95% 0.1M sulfuric acid Data UV-Vis: Concentration (ppm) 10 20 40 60 80 Unknown (Aspirin) Trial 1 Absorbance 0.189 0.342 0.798 1.278 1.719 1.774 Trial 2 Absorbance 0.193 0.340 0.813 1.281 1.744 1.774 Trial 3 Absorbance 0.196 0.333 0.816 1.275 1.751 1.778 Average Absorbance 0.193 0.337 0.809 1.278 1.738 1.775 UV-Vis: Concentration V. Absorbance y = 0.0225x - 0.0745 R² = 0.9978 Absorbance 2 1.5 1 0.5 0 0 20 40 60 80 100 Concentration (ppm) Fluorescence: Excitation Wavelength: 343nm Emission Wavelength: 449 Concentration (ppm) 1 2 3 Trial 1 Intensity 489.794 785.836 1016.026 Trial 2 Intensity 488.325 785.723 1016.026 Trial 3 Intensity 486.069 781.790 1016.026 Average Intensity 488.063 784.450 1016.026 4 5% Tonic Water Solution (Sterling Brand) 1016.026 1016.026 1016.026 1016.026 522.984 522.365 519.365 521.571 Fluorescence: Concentration V. Intensity y = 263.98x + 234.88 R² = 0.995 1200 Intensity 1000 800 600 400 200 0 0 0.5 1 1.5 2 2.5 3 3.5 Concentration (ppm) Calculations UV-Vis: Dilutions (1000ppm)(xmL)=10ppm X 25mL0.25mL stock solution in a 25mL volumetric flask and diluted to line with hexane (1000ppm)(xmL)=20ppm X 25mL0.5mL stock, 25mL volumetric flask, dilute to line with hexane (1000ppm)(xmL)=40ppm X 25mL1mL stock, 25mL volumetric flask, dilute to line with hexane (1000ppm)(xmL)=60ppm X 25mL1.5mL stock, 25mL volumetric flask, dilute to line with hexane (1000ppm)(xmL)=80ppm X 25mL2mL stock, 25mL volumetric flask, dilute to line with hexane Aspirin Sample: Average Absorbance is 1.775 𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒 = 0.0225(𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛) − 0.0745 1.775 = 0.0225(𝑋) − 0.0745 𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 = 82.2𝑝𝑝𝑚 Fluorescence: Dilutions (𝐼𝑛𝑖𝑡𝑖𝑎𝑙 𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑝𝑝𝑚)(𝑥 𝑚𝐿) = (𝐷𝑒𝑠𝑖𝑟𝑒𝑑 𝑝𝑝𝑚)(𝑉𝑜𝑙𝑢𝑚𝑒 𝑠𝑡𝑜𝑐𝑘 𝑤𝑖𝑙𝑙 𝑏𝑒 𝑑𝑖𝑙𝑢𝑡𝑒𝑑 𝑡𝑜) (1000𝑝𝑝𝑚)(𝑥 𝑚𝐿) = (100𝑝𝑝𝑚)(25𝑚𝐿) Ex 2.5𝑚𝐿 𝑜𝑓 1000𝑝𝑝𝑚 𝑖𝑛 𝑎 25𝑚𝐿 𝑣𝑜𝑙𝑢𝑚𝑒𝑡𝑟𝑖𝑐 𝑓𝑙𝑎𝑠𝑘, 𝑑𝑖𝑙𝑢𝑡𝑒 𝑡𝑜 𝑙𝑖𝑛𝑒 𝑤𝑖𝑡ℎ 0.1𝑀 𝐻2 𝑆𝑂4 During course of lab, ran out of 0.1M Sulfuric acid. Was reported that initial concentration of concentrated sulfuric acid was 18M (18𝑀)(𝑥 𝐿) = (0.5𝐿)(0.1𝑀) 2.778𝑚𝐿 𝑜𝑓 18𝑀 𝑠𝑢𝑙𝑓𝑢𝑟𝑖𝑐 𝑎𝑐𝑖𝑑 𝑖𝑛 𝑎 500𝑚𝐿 𝑣𝑜𝑙𝑢𝑚𝑒𝑡𝑟𝑖𝑐 𝑓𝑙𝑎𝑠𝑘 𝑎𝑛𝑑 𝑑𝑖𝑙𝑢𝑡𝑒 𝑡𝑜 𝑙𝑖𝑛𝑒 𝑤𝑖𝑡ℎ 𝑑𝑖𝑠𝑡𝑖𝑙𝑙𝑒𝑑 𝑤𝑎𝑡𝑒𝑟 5% tonic water solution: 50mL volumetric flask: 50 × 0.05 = 2.5𝑚𝐿 𝑡𝑜𝑛𝑖𝑐 𝑤𝑎𝑡𝑒𝑟 Plus 47.5mL 0.1M H2SO4 in the 50mL volumetric flask Intensity analysis of Tonic Water Solution: 𝐼𝑛𝑡𝑒𝑛𝑠𝑖𝑡𝑦 = 263.98(𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛) + 234.88 521.571 = 263.98(𝑋) + 234.88 𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 = 1.086𝑝𝑝𝑚 Conclusion As the experts on these two instruments for this lab section, this lab was a success. We determined how to use each instrument and found out about some pitfalls of each such as the spectrofluorometer having a tendency to turn itself off often. The procedure states to use 10, 5, 1, and 0.1ppm to create the calibration curve for the fluorescence part. But due to instrument constraints, that was not possible, so we determined the best course of action was to use 1ppm increments from 1ppm to 4ppm. Although it should be stated that something went wrong with the 4ppm standard in that it had the exact same intensity as the 3ppm standard. The error most likely occurred during the making of the four standards. Finally, upon completion of the calibration curves, we would be able to run a myriad of unknowns through each instrument to determine the concentration of, in this case salicylic acid, a compound in a solution. Additional raw data and graph from the UV-Vis can be found in the lab notebook. References Skoog, D.A., Holler, J.F., and Crouch, S.R. “Principles of Instrumental Analysis” Sixth Edition, Thomson Brooks/Cole, New York, 2007. pp. 132-135.