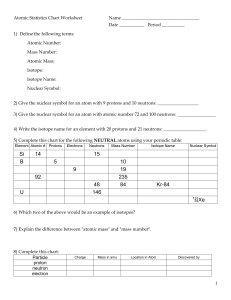
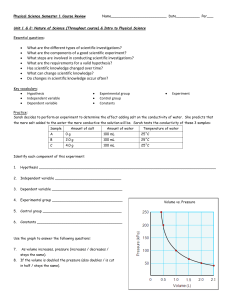
NAME___________________________________________PER
advertisement
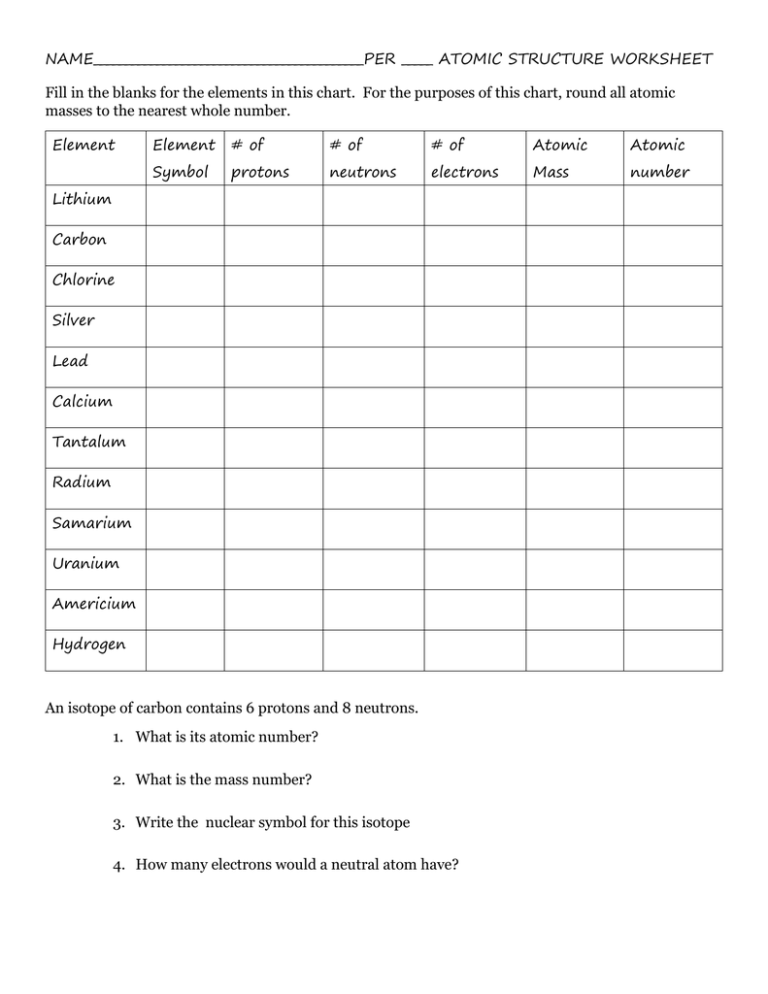
NAME___________________________________________PER _____ ATOMIC STRUCTURE WORKSHEET Fill in the blanks for the elements in this chart. For the purposes of this chart, round all atomic masses to the nearest whole number. Element Element # of # of # of Atomic Atomic Symbol protons neutrons electrons Mass number Lithium Carbon Chlorine Silver Lead Calcium Tantalum Radium Samarium Uranium Americium Hydrogen An isotope of carbon contains 6 protons and 8 neutrons. 1. What is its atomic number? 2. What is the mass number? 3. Write the nuclear symbol for this isotope 4. How many electrons would a neutral atom have? 5. Complete the table Nuclear Symbol # Of Protons 4 2𝐻𝑒 # Of Neutrons 2 2 6 8 # Of Electrons Atomic Number Mass Number 12 6𝐶 16 8𝑂 8 17 54 26𝐹𝑒 30 26 6. Which of the pairs of atoms in the table above are isotopes of the same element? (Write them using the nuclear symbol) 7. ___________________ Model of the Atom describes the atom as a positive ball with spots of negative embedded inside. 8. Describe the atom from John Dalton’s point of view. 9. Is O3 an element or a compound? __________________ 10. What is one evidence of the Big Bang Theory?_____________________________ ____________________________________________________________ 11. Write in scientific notation 60,557,000 ____________________ 12. Is a cup of Kool-Aid a homogeneous mixture or heterogeneous mixture?