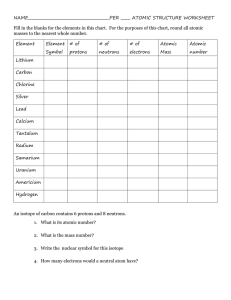
Atomic Statistics Chart Worksheet Name __________________________________ Date ___________ Period __________ 1) Define the following terms: Atomic Number: Mass Number: Atomic Mass: Isotope: Isotope Name: Nuclear Symbol: 2) Give the nuclear symbol for an atom with 9 protons and 10 neutrons: __________________ 3) Give the nuclear symbol for an atom with atomic number 72 and 100 neutrons: _________________ 4) Write the isotope name for an element with 20 protons and 21 neutrons: ___________________ 5) Complete this chart for the following NEUTRAL atoms using your periodic table: Element Atomic # Protons Si B Electrons 14 Neutrons Mass Number Isotope Name 10 19 235 84 Kr-84 Nuclear Symbol 15 5 9 92 48 146 U 131 54Xe 6) Which two of the above would be an example of isotopes? 7) Explain the difference between "atomic mass" and "mass number". 8) Complete this chart: Particle proton neutron electron Charge Mass in amu Location in Atom Discovered by 1 Atomic Statistics Chart Worksheet Name __________________________________ Date ___________ Period __________ Definitions: 1) ___________________ Represents the number of protons in an atom’s nucleus. 2) ___________________ Atoms of the same element with different masses. 3) ___________________ Total of protons and neutrons in an atom. 4) ___________________ Weighted average of the different isotopic masses of an element’s atoms 5) ___________________ Subatomic particle with a mass of 1 AMU and a charge of +1 6) ___________________ Subatomic particle with a mass of 0 AMU and a charge of –1 7) Complete this chart for the following NEUTRAL atoms using your periodic table: Element Nuclear Symbol Electrons Protons Neutrons Isotope Name Atomic Number Mass Number Xenon 133 Iodine 73 50 70 79 200 80 11 200 11 8) Copper has 2 main isotopes, Cu-63 and Cu-65. Look up copper's atomic mass on the periodic table. a) Which isotope, Cu-63 or Cu-65, is most abundant in nature? b) Explain how you can tell. 9) Boron has 2 main isotopes, Boron-10 and Boron-11. Which isotope is more abundant in nature and how can you tell? 2