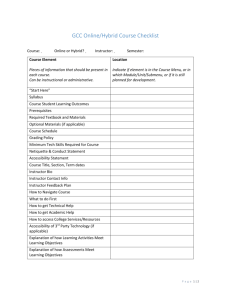
Integration Architecture
advertisement

Hybrid systems methods for
biochemical networks
Adam Halasz
Outline
• Hybrid systems, reachability
• Piecewise affine approximations of biochemical
systems
• Example I: Glucose-lactose
• Example II: Tetracyclin resistance
Biomolecular networks as hybrid systems
Networks of chemical and molecular processes
State = {values of all concentrations}
Rates of each process are continuous functions of the state
Several layers of processes, different timescales
State space can be huge (O(103) variables for one cell)
Lots of truly discrete behavior:
Genes on/off
Discrete variables
Lots of apparent discrete behavior
Nontrivial continuous dynamics produces multistability, bifurcations
Abstractions – commonly used and/or required for simplification
Biomolecular dynamical systems
Central dogma of molecular
DNA encodes genes; it replicates
Genes are transcribed into mRNA
mRNA is translated into proteins
biology
Proteins may:
act as enzymes that catalyze metabolic reactions
act as transcription factors
Metabolic reactions
big network that converts incoming nutrients into useful
substances and by-products
reactions proceed much faster when the right enzymes are
available
The Central Dogma
•DNA replicates during cell division
•Transcription performed by RNA
polymerase
•Requires a promoter site
•Several genes bundled to one
promoter = operon
•In higher organisms, mRNA is spliced
•Translation performed by Ribosomes
•Protein synthesis needs raw material
Genes to proteins
• Proteins are synthesized as
chains of elementary
proteins, amino-acids
• They fold, giving rise to
complicated 3d structures
• Several molecules may be
assembled into more
complicated ‘machines’, such
as RNAP, ribosomes, etc.
Metabolic network
•Very complex
•Structured
•Stoichiometry is more easily
identified than rate laws
•Many networks available in
databases, e.g. Kegg
•Reactions linked to individual
genes
•Lots of feedback
Metabolic network has a lot of control
• Feedback between
Metabolites
Genes and proteins
• Continuous adjustment to external
conditions
• Signaling networks
• Control is through rate laws, but also
through stochastic mechanisms
Hybrid systems
• much of the underlying dynamics is continuous, but..
• complexity and lack of detailed kinetic information require
the use of hybrid abstractions
Hybrid systems
Two topics to be addressed:
1.
How to build a good hybrid abstraction
2.
How to analyze a network that includes
hybrid abstractions
Using hybrid systems abstractions to build
hybrid systems abstractions
•The lac operon is a bistable genetic switch
Multiple positive feedback bistable
Input: external lactose
State: x={M,B,A,L,P}
x f (x)
mRNA
β-gal
perm
repressor
AlloLactose
Lactose
External
Lactose
Using hybrid systems abstractions to build
hybrid systems abstractions
•May be abstracted to an automaton:
Input: external lactose
State: {I}
Le Llow
e
HIGH
I=1
LOW
I=0
Le Lhigh
e
Llow
f (...); Lhigh
g (...)
e
e
The characteristic still depends on
the underlying kinetic parameters!
Reachability
•The full lac model can be simulated to investigate induction, but that can be
expensive
•The question of whether induction is possible may be framed as a reachability
problem
•Many other situations with discrete outcomes are amenable to reachability
Initial
Question 1:
which ones end up in a viable
final state?
Final
Irreversible
damage
Question 2:
which ones survive?
Kinetics 1
Dynamic models have a special structure!
Example
A B C
d A
k r C k f AB
dt
d B
k r C k f AB
dt
d C
k f AB k r C
dt
More generally,
x f l (x)
f l ( x)
iN
i1
l
c
x
x
i1 ,,iN 1 N
i1 ,i N {0 ,1}
Kinetics 1 (continued)
y
y e gy1 f hy1 x
x a cy1 b dy1 x
y e gy1 f hy1 x
y1
x
x a bx cy dxy
y e fx gy hxy
The vector field is a
unique (affine) function
of the vectors at the end
points
Kinetics 1 (continued)
x f (x)
The vector field is a unique function
of the vectors at the vertices
[Belta, Habets, Kumar 2002]
Transcription rate
Kinetics (2)
Hybrid System
Rectangular partitions
Affine dynamics
Transcription rate
Concentration of repressor
Concentration of allolactose
Piecewise affine approximation
Vmax [ S ]
r ([ S ])
K m [S ]
Vmax [ S ]
[S ] 2K m
r ([ S ]) 2 K m
Vmax
[S ] 2K m
Simplest approximation with two affine pieces
Can use any number, to achieve any desired precision
Piecewise is hybrid
Vmax [ S ]
[S ] 2K m
r ([ S ]) 2 K m
Vmax
[S ] 2K m
[S ] 2K m
[ P ]
Vmax
[S ]
2K m
[ P ] Vmax
[S ] 2K m
Piecewise approximation has different equations in each interval
Transitions occur as the variable switches intervals
Several substrates that
saturate
S2
[ P1 ] B[ S1 ] ; [ P2 ] C
[ P1 ] B[ S1 ]
[ P ] D[ S ]
2
2
[S1 ] T1
[ P1 ] A ; [ P2 ] C
[S1 ] T1
[S2 ] T2
[S2 ] T2
T2
[ P1 ] B[ S1 ] ; [ P2 ] D[ S2 ]
[S1 ] T1
S1
[S2 ] T2
[ P1 ] A ; [ P2 ] D[ S2 ]
[S1 ] T1
[S2 ] T2
T1
Piecewise approximation has different equations in each interval
Transitions occur as the variable switches intervals
Can continue in many dimensions
Abstraction
Model the biochemical network as a switched system
with continuous multi-affine dynamics
Each mode has simple dynamics
More insight
Approximation may be refined as needed
Partition may be refined independently of dynamics
No additional computational difficulties
Traditional simulations are easier
Efficient reachability algorithms can be applied
Reachability analysis
Can the system reach a set of states starting from a set
of initial conditions?
x2sw1
Analysis
x2sw 2
x3
x3sw 2
x3sw1
x2
x1sw1
x1sw 2
x1
x2sw1
Analysis
x2sw 2
x3
x3sw 2
x3sw1
x2
Initial
x1
Reachable
x1sw1
x1sw 2
Unreachable
Hybrid System Analysis
Reachability
Cell A is reachable from
cell B if there is at least
one trajectory from B to A
Cell A is not reachable
from cell B if there are no
trajectories from B to A
Glucose-lactose system
• The lactose metabolism is self-nourishing:
The cell needs enzymes for:
Inbound lactose transport (permease)
Lactose processing (ß-galactosidase)
Permease
and ß-galactosidase are gene
products of the lac operon
Lac operon is repressed in the absence of
allolactose
Allolactose is produced when lactose is
processed
• Bistability:
a
low and a high lactose metabolism state
induction needed to move into the high state
Lac system in E.coli
mRNA
β-gal
perm
repressor
AlloLactose
Lactose
External
Lactose
Lac system in E.coli
Crucial switching property, sensitive to basal rate
Can be framed in terms of reachability
Lac system in E.coli
Hybrid model constructed using a fine grained linearization of the
nonlinear rate laws
Predictions of the two models are very similar
Hybrid model within 5% uncertainty of model parameters
Glucose-lactose system
• Lactose is an alternative energy source
• Glucose is the preferred nutrient; bacteria
also grow on lactose, but only when
glucose is absent
• There are two mechanisms that ensure this:
Inducer
exclusion
Catabolite repression
mRNA
Lac
repressor
perm
b-gal
AlloLactose
Lactose
External
Lactose
Glucose inhibits
the influx of
lactose
CAP
cAMP
CAP competes with lac
repressor, enhancing
transcription
External
Glucose
cAMP is produced
when glucose is
absent
Steady states
• For a given Glucose (Ge) value, the steady state line is S-shaped
• The bistable section increases as Ge increases
• The upper threshold for Lactose (Le) is higher if Ge is present
Induction and reachability
Expect the vicinity of zero to be confined when system is bi-stable
… unless it is induced by increasing
Le, decreasing Ge, or both
Suppose initially the system is at
zero allolactose. Then it will have to
settle on the lower sheet..
Induction and reachability
Up-switching possible if (Le,Ge) outside the bistable region
for some time
Upward switching trajectories
Final, induced state
Initial state, close to zero
Induction and reachability
B
Follow trajectories in state space
Induced trajectories leave the vicinity of the initial
state
A
Induction and reachability
B
Cover the area of interest with a grid
A
Induction and reachability
B
Induced trajectories leave the vicinity of the initial state
For reachability, only need to cover the vicinity
Verify those configurations that do not leave the grid
A
Discretization
Reachability results
• Calculate highest Allolactose (A) reached
• Sweep for (Le,Ge)
Bistable regions are
non-inducible,
hence they reach
only the lower A
values
Reachability results
Non-inducible region should match the footprint of
bi-stability
Reachability results
Analyzing networks of hybrid
abstractions
• The lac switch is one piece in a potentially huge circuit,
which has both discontinuous and continuous elements
• A “true” of hybrid system:
Discontinuous dynamics
Different state variables
Filippov states!
Hierarchy of modes!
Networks of hybrid abstractions
•Continuous part of state space is still a set of concentrations
•Dynamics is still given by reaction rates
•Reaction rates are given by discontinuous functions of the state
variables:
x f (x)
Networks of hybrid abstractions
• Partition of continuous part of state space along threshold
values
• Boundaries treated as separate modes
• Discrete transition system
• Model checking
Networks of hybrid abstractions
• Can analyze complex interconnections
• Elucidate roles of genes
Summary
• Molecular biology offers many instances of
‘natural’ hybrid systems
• Very large state spaces, thousands of substances
• Complex networks, nonlinear equations
• Switching and other discontinuous behavior
Genes on/off
Multistability, bifurcation
Hybrid abstractions
• Two aspects:
Constructing hybrid abstractions
Analyzing networks a hybrid systems
• Both directions work towards automated analysis
Reading
•
•
•
•
•
•
Calin Belta – Boston U.
Hidde de Jong – INRIA Rhone-Alpes, FR/EU
Claire Tomlin – Berkeley
Ashish Tiwari – SRI, Palo Alto, CA
Joao Hespanha – Santa Barbara
V. Kumar, O. Sokolsky, G. Pappas, A. Julius, A.
Halasz – U. Penn
• Hybrid systems, reachability
• Piecewise affine approximations of biochemical
systems
• Example I: Glucose-lactose
• Example II: Tetracyclin resistance
Tetracycline resistance via TetA efflux
Tc0
periplasm
efflux
diffusion
Mg
Tc
cytoplasm
[TcMg]+
tetR
O1 O2
tetA
TetA
TetR
[TcMg]+TetR
Tc0
periplasm
efflux
diffusion
Mg
cytoplasm
Tc
[TcMg]+
tetR
O1 O2
TetR
[TcMg]+TetR
tetA
TetA
Tet Model Analysis
• Model describes a bacterial defense mechanism against
attack with an antibiotic (tetracycline, Tc)
• Tc destroys the cell’s ribosomes, inflicting potentially
irreversible damage to the transcription-translation
apparatus.
• Objective is to avoid accumulation of Tc inside the cell.
• Our objective: to disrupt the defense mechanism. For this
we first have to:
1.
2.
Assess the actual model parameters
Identify parameter modifications that disrupt the mechanism
Tet Model Building
• Model parameters not fully known
Use
existing information on known reactions
Use
consistency checks and qualitative
arguments
Determine
parameters indirectly by comparing
model predictions to experimental results
• Perform experiments to verify model
Tet Model Analysis
Measures of the defense
mechanism’s effectiveness:
•Irreversible damage to
transcription-translation
apparatus:
Direct investigation would
require a greatly expanded
model
Use proxies instead
•Final Tc concentration
May not tell the whole story
•Maximum transient Tc
concentration
May cause irreversible
damage
Tet Model Analysis
•We wish to investigate how these efficiency measures
depend on model parameters, especially those parameters
that are not well known.
•We may indirectly pin down their value ranges
•We may learn which aspects of this mechanism are the
most easy to compromise by targeting with a drug
•Final Tc concentration
Computed by a steady state calculation
•Maximum transient Tc
Not directly calculable
One way: many simulations
Other method: reachability
TetModel Use Case (2005)
Model
CHARON
SBML2Charon
UPenn
SBMLEditor
Reachability
Tools
Parameter
ranges
Equilibrium
Point Analyzer
Full Model
(SBML,
annotated)
Hybrid System
Model Builder
(HSMB)
UPenn
Hybrid Model
(SBML)
Simulator
UPenn
Stochastic
Simulator
UTenn
Experimental
Traces
ODE
Simulator
UPenn
Simpathica
Toolset
NYU
Hybrid SAL
SRI
Validation of hybrid
system abstractions
Summary
• Hybrid systems bridge the gap between discrete “big
picture” models and detailed, continuous dynamics
• Piecewise multi-affine approximations are well suited for
biochemical networks
• Several software tools apply efficient algorithms for:
Model building
Simulation
Reachability
• Type of problems:
Mid-sized networks, focus on one mechanism
Analysis of parameter and initial state ranges
Prediction of qualitative outcomes
Stringent response
The stringent response is the set of metabolic and
regulatory changes that take place in a bacterium as a
consequence of a downshift in the availability of
nutritional substances, especially amino-acids.
Transcription is globally decreased
Promoters for stable RNA are downregulated
Promoters for amino-acids are upregulated
Stringent response
Stringent response
Model block diagram
Stalled complexes
tRNAc,u
(p)ppGpp
(p)ppGpp
reactions
Upregulated mRNA
Translation
Downegulated mRNA
Transcription
Ribosome
assembly
proteins
Ribosomes
Ribosomal RNA
Stringent response
Hybrid model with 9 variables, 2 modes
One outside control (amino-acid availability)
Negative feedback, only one steady state for given
conditions
Stringent response
Steady-state calculations
Signaling substance increases with parameter r
Transcription [initiation] rate decreases
Stringent response
Dynamic calculations
Surge of signaling substance indicates potentially lethal
condition: excessive accumulation of stalled transcriptional
complexes
Reachability analysis can constrain the peak value