Catalyst - Classteacher
advertisement

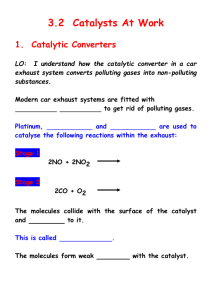
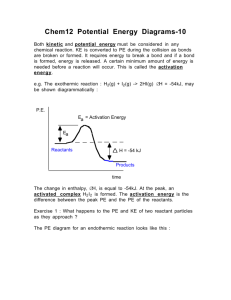
Catalysts Learning Objectives Discuss types of catalyst Differentiate between homogeneous and heterogeneous catalyst Describe properties of catalyst Study theories of catalysis Catalyst A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of catalyst as you had at the beginning. Catalysis is the change in rate of a chemical reaction due to the participation of a catalyst Some examples Fe Reaction Catalyst Decomposition of hydrogen peroxide Manganese(IV) oxide, MnO2 Nitration of benzene Concentrated sulphuric acid Manufacture of ammonia by the Haber Process Iron Conversion of SO2 into SO3 during the Contact Process to make sulphuric acid Vanadium(V) oxide, V2O5 Hydrogenation of a C=C double bond Nickel Types of catalyst Catalysts can be divided into two main types – heterogeneous and homogeneous i) Homogeneous catalyst - the catalyst is in the same phase as the reactants. Example- The reaction between persulphate ions and iodide ions. Fe2+ S2O82- + 2I2 SO42- + I2 ii) Heterogeneous catalyst - the catalyst is in a different phase from the reactants. Example- The hydrogenation of a carbon-carbon double bond. CH2=CH2 + H2 Ni CH3-CH3 Properties of catalyst Positive Catalysis The catalyst which increases the rate of a chemical reaction is called positive catalyst and the phenomenon is known as positive catalysis Examples are MnO2 (i) 2KClO3 2KCl + 3O2 Pt (ii) H2O2 H2O + [O] Negative Catalysis The catalyst which decreases the rate of reaction is called negative catalyst and phenomenon is called negative catalysis Examples are Acetanilide (i) H2O2 H2 O + O (ii) Knocking of petrol by tetraethyl lead Properties of catalyst Promotors Sometimes the activity of a catalyst may be increased by addition of small amount of a second substance. The second substance, which though itself is not a catalyst, promotes the activity of a catalyst, is called the promoter For example, Fe ,Mo/ Al2O3 N2 + 3H2 2NH3 [Fe] is the catalyst and presence of Mo or Al2O3 increase the activity of iron and, therefore, acts as promoter. Properties of catalyst Poison Sometimes the rate of a catalysed reaction is reduced by the presence of a small amount of same substance (may be as impurities in the reactants). Such a substance, which destroys the activity of a catalyst, is called poison and the process is called catalytic poisoning For example, in reaction Pt ,CO 2H2 + O2 2H2O Activity of catalyst Pt is poisoned by presence of CO Fe ,H2S N2 + 3H2 2NH3 Presence of H2S reduces the activity of Fe Theories of catalysis The two main theories of catalysis are: (i) intermediate compound formation theory and (ii) adsorption theory I) Intermediate Compound Formation Theory According to this theory, the catalyst reacts with one of the reactants to give an intermediate, which reacts with another reactant to yield products and the catalyst as follows: A + [ Catalyst] [Intermediate] [Intermediate] + B Product + [Catalyst] Examples: [NO] 2 SO2 + O2 Proceeds as: 2NO + O2 NO2 + SO2 2 SO3 2NO2 SO3 + NO II) Adsorption Theory The heterogeneous catalysis e.g. gaseous reaction on a solid surface, is explained by this theory as follows: Catalyst A(g) + B(g) C(g) +D(g) Following four steps are involved in the heterogeneous catalysis: (i)Diffusion of reactants at the surface of the catalyst. (ii)Adsorption of reactants at the surface. (iii)Reaction of reactants at the surface. (iv)Desorption of products from the surface.