Chapter 4 CARBON AND THE MOLECULAR DIVERSITY OF LIFE
advertisement
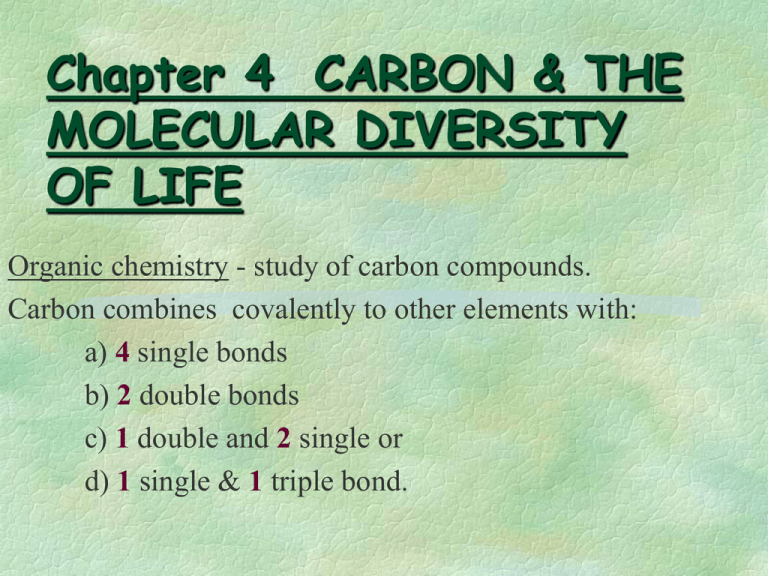
Chapter 4 CARBON & THE MOLECULAR DIVERSITY OF LIFE Organic chemistry - study of carbon compounds. Carbon combines covalently to other elements with: a) 4 single bonds b) 2 double bonds c) 1 double and 2 single or d) 1 single & 1 triple bond. Objectives: Organic Chemistry is the study of carbon compounds Variation in carbon skeletons leads to diversity of organic molecules Functional groups contribute to the diversity of life Root Words Con – Di – Glyco – Hydro – - lyse Macro – Meros – Mono – - sacchar Poly – Tri – Functional Groups: •Not C-H Bonds. •Region in molecule where reactions take place. •All are “Hydrophilic”. (JUST THE GROUP, not necessarily the entire molecule). •Increases the solubility of the compound. •Determine the properties of a carbon molecule. Table 4-1 Functional Groups of Organic Compounds NOTE: Carboxyl group is a source of H ions (Protons) so it is an acid, since acids are a source of electrons in solution Amino group picks up H ions (protons) in solution so this makes it a base. So what is an organic acid? Functional Group Activity Directions: Using page 58 in your text, create flash cards for the functional groups. Functional Group will go on the front, and structure, name and example on the back. You may want to color code them. VALENCES OF MAJOR ELEMENTS IN ORGANIC MOLECULES: (Fig. 4.3) NOTE: Carbon is the most abundant element by dry weight in all living things. The Shapes of 3 Simple Organic Compounds: (Fig. 4.2) •Hydrocarbons: C & H Compounds. •(Fig. 4.4) Hydrocarbons-Nonpolar; Hydrophobic thus not soluble in water. Polar molecules: Example - polar compound HCl H+ & Cl-. Remember Isomeres are compounds that have the same written formula but have different structural formulas. (Fig.4.6) Fig. 4.7