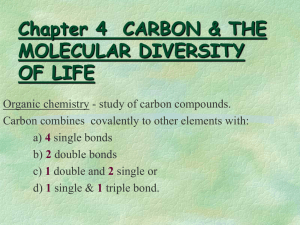Introduction to Organic Chemistry
advertisement

Organic chemistry is the study of carbon-containing compounds (especially compounds containing C-C bonds) The field of organic chemistry is very important for a wide variety of reasons. A huge number of carbon-containing compounds are known. More than 16 million known compounds About 90% of new compounds made each year contain carbon Most of the advances in the pharmaceutical industry are based on a knowledge of organic chemistry. Many drugs are organic compounds Life as we know it is based on organic chemistry. Most biologically important compounds contain carbon: DNA, RNA proteins carbohydrates Organic compounds are those compounds found in any organism that is living or was once living containing carbon. Compounds lacking carbon and not from living organisms referred to as “inorganic”. Organic compounds are those compounds found in any organism that is living or was once living containing carbon. Most organic compounds have a “skeleton” that is composed of C-C bonds. The C-C bonds may be single bonds, double bonds, or triple bonds. The “skeleton” of an organic compound has H’s attached to it. other “heteroatoms” like O, halogens or N may be present as well The number of bonds formed by C in an organic compound is determined by the electron configuration of C. Carbon has four valence electrons: 1s22s22p2 Carbon generally forms 4 equivalent bonds. The formation of four equivalent bonds is best explained using the concept of hybrid orbitals. Hybrid orbitals When C forms four single bonds: sp3 hybrid orbitals are involved tetrahedral geometry When C forms a double bond: sp2 hybrid orbitals are used trigonal planar geometry When C forms a triple bond: sp hybrid orbitals are used linear geometry Organic compounds contain not only C-C bonds but also C-H bonds. C-C and C-H bonds tend to be non-polar because there is a small difference in electronegativites Most (but not all) organic compounds are relatively non-polar generally not very soluble in water When we write a simple chemical formula, such as CH4, we are actually writing what we call a molecular formula Molecular Formulas – show the atoms and the number of atoms involved in a molecule but nothing else In organic chemistry, it is often more useful to show structural formulas instead Structural Formulas – show each type of atom and how they are arranged in a molecule H CH4 H - C - H H Molecular Formula Structural Formula 3-D Structure Structural formulas are important in organic chemistry because of isomers Isomers are two compounds with the SAME MOLECULAR formulas but different structural formulas -- they have different chemical and physical properties C3H8O The simplest organic compounds are the hydrocarbons: organic compounds that contain only carbon and hydrogen four general types: alkanes alkenes alkynes aromatic hydrocarbons Alkanes: hydrocarbons that contain only single bonds Examples: Methane Ethane CH4 C2H6 Alkenes: hydrocarbons that contain a C = C double bond H2C = CH2 (ethylene) Alkynes: hydrocarbons that contain a C bond H – C C triple C – H (acetylene) There are others to follow but these are the first three simplest organic molecules. Organic compounds that are soluble in polar solvents such as water generally have a polar functional group present in the molecule. An atom or group of atoms that influences the way the molecule functions, reacts or behaves. An atom or group of atoms in a molecule that undergoes predictable chemical reactions The center of reactivity in an organic molecule Since functional groups are responsible for the many of the chemical and physical properties of organic compounds, we often classify and study organic compounds by the type of functional group present. Functional Type of Group Compound C=C alkene C alkyne C Example H2C = CH2 HC CH C–O–H alcohol CH3OH C–O–C ether CH3CH2OCH2CH3 C–N amine CH3NHCH3 Functional Group Type of Compound O C–H O aldehyde O C–C–C Example CH3C – H O ketone CH3 – C – CH3 Functional Group O Type of Compound Carboxylic C–O–H acid O C–O–C Example O CH3C – O – H O ester CH3C – O – CH2CH3 Example: Name the functional groups that are present in the following compounds: O CH3CH2OH H2C = CHCOH CH3CH2NCH3 CH3 1)Acyclic—contains no rings in the structure 2)Carbocyclic—contains at least one ring only made up of carbon 3)Heterocyclic—contains at least one ring that has an element other than carbon in it acyclic carbocyclic heterocyclic