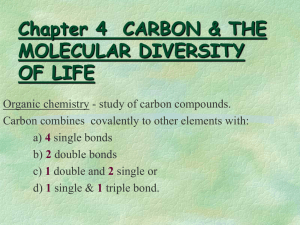03. hydroc.funcgrps.doc

D’YOUVILLE COLLEGE
BIOLOGY 102 - INTRODUCTORY BIOLOGY II
LECTURE # 3 ORGANIC CHEMISTRY I
1. Organic Compounds:
• organic chemistry: chemistry of carbon-based compounds
- carbon’s valence (+ or – 4) facilitates formation of immense variety of compounds of different sizes & shapes
- carbon bonds mainly with hydrogen, oxygen & nitrogen (fig. 4 – 4 & ppt. 1)
• hydrocarbons: non-polar, hydrophobic compounds consisting of carbon and hydrogen only (fig. 4 – 3 & ppt. 2)
- chains of variable length, straight or branched or cyclic, saturated or unsaturated, e.g. propane, decane, propene, etc. (fig. 4 – 5 & ppt. 3); neutral fats or oils are main hydrocarbons of living systems
- neutral fats - non-polar (hydrophobic) substances due to high hydrocarbon content (fig. 4 – 6 & ppt. 4)
• isomers – compounds that share same number & type of atoms, but differ in branch
points, position of double bonds, etc. (fig. 4 – 7 & ppt. 5)
- different isomers may have profoundly different properties due to shape differences
(fig. 4 – 8 & ppt. 6)
Biology 102 - Spring ‘09 page 2
• functional groups: chemical groupings including atoms other than carbon & hydrogen, e.g., oxygen & nitrogen
- these groupings confer important properties upon the compounds in which they are found (ppt. 7)
- specific groups added to basic hydrocarbon structure generate new families of compounds with different properties (fig. 4 – 9, ppts. 8 & 9):
- hydroxyl (alcohols) e.g., ethanol
- carbonyl (aldehydes & ketones) e.g., formaldehyde, acetone
- carboxyl (organic acids) e.g., fatty acids
- amino (amines – organic bases) e.g., amino acids
- sulfhydryl (thiols) e.g., cysteine (sulfur-containing amino acid)
- phosphate (organic phosphates) e.g., phospholipids
- methyl (methylated compounds) e.g., sex steroids, N-bases in nucleic acids