Chapter 8 Lecture

Nucleophilic Substitution
–
Y :
+
R
Nucleophilic Substitution
X Y R + : X
–
Nucleophile is a Lewis base (electron-pair donor), often negatively charged and used as
Na + or K + salt.
Substrate is usually an alkyl halide.
Effect of Hybridization of the Carbon
Halogens connected to sp 2 carbons are not reactive under the conditions discussed in this chapter. sp 3 carbon reactive sp 2 carbons not reactive
Nucleophilic Substitution
Therefore, substrate cannot be an a vinylic halide or an aryl halide, except under certain conditions to be discussed in
Chapter 12.
Table 8.1 Examples of Nucleophilic Substitution
Alkoxide ion as the nucleophile
R'
..
–
..
: + R X gives an ether
R'
..
R + : X
–
Example
(CH
3
)
2
CHCH
2
O Na + CH
3
CH
2
Br
Isobutyl alcohol
(CH
3
)
2
CHCH
2
O CH
2
CH
3
+ Na Br
Ethyl isobutyl ether (66%)
Table 8.1 Examples of Nucleophilic Substitution
Carboxylate ion as the nucleophile
O
R'C
..
–
..
: + R X gives an ester
O
R'C
..
R + : X
–
Example
O
CH
3
(CH
2
)
16
C O K + CH
3
CH
2
I acetone, water
CH
3
(CH
2
)
16
O
C O CH
2
CH
3
+ K I
Ethyl octadecanoate (95%)
Table 8.1 Examples of Nucleophilic Substitution
Hydrogen sulfide ion as the nucleophile
H
..
–
S : + R X gives a thiol
H
..
R + : X
–
Example
K S H + CH
3
CH(CH
2
)
6
CH
3
Br ethanol, water
CH
3
CH(CH
2
)
6
CH
3
+ K B r
S H
2-Nonanethiol (74%)
Table 8.1 Examples of Nucleophilic Substitution
Cyanide ion as the nucleophile
: N C
–
: + R X gives a nitrile
:
N C R + : X
–
Example
NaCN +
DMSO
CN + Na Br
Cyclopentyl cyanide (70%)
Br
Table 8.1 Examples of Nucleophilic Substitution
Azide ion as the nucleophile
:
– + –
..
N
..
: + R X gives an alkyl azide
:
– +
..
N
..
R + : X
–
Example
Na N
3
+ CH
3
CH
2
CH
2
CH
2
CH
2
I
2-Propanol-water
CH
3
CH
2
CH
2
CH
2
CH
2
N
3
+ Na I
Pentyl azide (52%)
Table 8.1 Examples of Nucleophilic Substitution
Iodide ion as the nucleophile
..
–
:
..
: + R X gives an alkyl iodide
:
..
R + : X
–
Example
CH
3
CHCH
3
+ Na I
Br acetone
CH
3
CHCH
3
+ Na Br
I
63%
NaI is soluble in acetone;
NaCl and NaBr are not soluble in acetone.
Relative Reactivity of Halide Leaving Group
Alkyl iodides are most reactive since:
(a) the carbon-iodine bond is weakest for the halogens;
(b) iodide is the weakest base of the halides (this implies that iodide is most stable) since HI is the strongest acid.
S
N
2 Mechanism of Nucleophilic Substitution
Since the rate of reaction depends on the halide the rate determining step must involve breaking of the carbonhalogen bond.
Kinetic studies of the above reaction showed that the rate is also dependent on the hydroxide concentration:
The rate determining step therefore involves both the nucleophile and the alkyl halide.
S
N
2 Mechanism of Nucleophilic Substitution
Overall reaction.
The mechanism (one step).
The transition state.
The configuration at carbon.
From
Potential Energy Diagram for S
N
2 Reaction
Inversion of Stereochemistry with S
N
2
The nucleophile attacks carbon from the side opposite the bond to the leaving group. The S
N
2 reaction at a chirality center proceeds with inversion of configuration at the carbon bearing the leaving group.
Inversion of Configuration
The reaction of (S)-2-bromooctane proceeds with inversion of configuration as shown in the equation:
The transition state is:
Steric Effects and S
N
2 Reaction
Rates
Effect of the Alkyl Group on the Rate
The rate of the reaction:
RBr + Li I R I + LiBr decreases with increasing steric hindrance.The reaction is fastest with the least hindered methylbromide.
The rate of S
N
2 reactions normally decreases in the order:
CH
3
X > primary > secondary > tertiary
Effect of b
-Substitution on the Rate
Substitution on the b
-carbon also slows reaction by adding steric hindrance to the carbon bearing the halogen.
Neopentyl bromide (a primary alkyl halide) is so hindered that it is essentially unreactive.
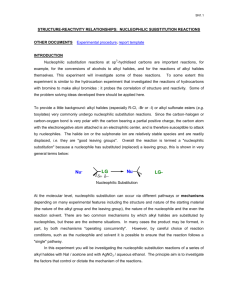
Nucleophiles and Nucleophilicity
Neutral Nucleophiles
Not all nucleophiles are negatively charged. Amines
(R
3
N), sulfides (R
2
S) and phosphines (R
3
P) are good neutral nucleophiles.
Neutral Nucleophiles
Solvolysis reactions with water (hydrolysis) and alcohols also involve neutral nucleophiles
S
N
2 hydrolysis reaction mechanism:
Nucleophilic Strength
Nucleophilicity (nucleophile strength) is a measure of how fast a Lewis base displaces a halogen in a reaction.
The table compares rates of reaction with CH
3
I in CH
3
OH.
Nucleophilicity
When comparing nucleophiles with the same nucleophilic atom then the stronger base is the stronger nucleophile.
The stronger base is the conjugate base of the weaker acid.
This generalization holds when comparing nucleophiles in the same row of the Periodic table
Nucleophilic Strength
When comparing nucleophiles that have the same nucleophilic atom the charged nucleophile is stronger.
Nucleophilic Strength
When comparing nucleophiles in the same group of the Periodic Table the most important factor is solvation of the nucleophile.
Iodide is the weakest base of the halogens but the best nucleophile. The smaller chloride is a stronger base but is more solvated because it has higher charge density.
The S
N
1 Mechanism of
Nucleophilic Substitution
A question...
Tertiary alkyl halides are very unreactive in substitutions that proceed by the S
N
2 mechanism.
Do they undergo nucleophilic substitution at all?
Yes. But by a mechanism different from S
N
2.
The most common examples are seen in solvolysis reactions.
Nucleophilic Substitution of Tertiary Alkyl halides
The reaction
(CH
3
)
3
CBr + 2 H
2
O (CH
3
)
3
COH + H
3
O + + Br follows a first order rate law:
Rate = k [(CH
3
)
3
CBr]
And the reaction is termed S
N
1.
S
N
1 Mechanism of Nucleophilic Substitution
Step 1: Ionization to form a tertiary cation.
Step 2: Addition of a water molecule.
Step 3: Deprotonation.
Potential Energy Graph of the S
N
1 Reaction
Characteristics of the S
N
1 mechanism first order kinetics: rate = k[RX] unimolecular rate-determining step carbocation intermediate rate follows carbocation stability rearrangements sometimes observed reaction is not stereospecific much racemization in reactions of optically active alkyl halides
The Alkyl Halide and the Rate of S
N
1 Reaction
Comparison of a series of alkyl bromides under S
N
1 reaction conditions (solvolysis) reveals that tertiary alkyl halides react fastest.
In general, methyl and primary alkyl halides never react by the S
N
1 mechanism and tertiary alkyl halides never react by S
N
2.
Stereochemistry of the S
N
1 Reaction
S
N
1 reactions proceed through the carbocation which is planar.
The nucleophile can react from either side however, surprisingly a 1:1 mixture of products is not always formed.
Stereochemistry of the S
N
1 Reaction
The incomplete loss of stereochemistry is explained by a partial shielding of one side of the cation by the halide leaving group.
Carbocation Rearrangements in S
N
1 Reactions
Rearrangements are evidence for carbocation intermediates and serve to confirm the S
N
1 reaction mechanism.
Mechanism with Rearrangement
Step 1: Ionization.
Step 2: H-shift (to form a more stable tertiary cation).
Step 3: Water addition to the carbocation.
Step 4: Deprotonation.
Effect of Solvent on Substitution
Solvent affects the rate of a reaction not the products formed and the questions are:
1. What properties of the solvent influence the rate most?
2. How does the rate-determining step of the mechanism respond to the properties of the solvent?
Classification of Solvents
Protic solvents are those that are capable of hydrogen bonding. Normally they have an –OH group or an N-H group.
Aprotic solvents are not hydrogen bond donors.
Polarity of a solvent is related to its dielectric constant ( e
).
Solvents with high dielectric constants are considered polar and those with low dielectric constants are non polar.
Properties of Solvents
Solvents and S
N
2 Reactions
Polar protic solvents hydrogen bond to, and solvate, the nucleophile and suppress its nucleophilicity and reduce the rate of reaction.
Polar aprotic solvents cannot solvate the nucleophile and the nucleophile is free to react.
Solvents and S
N
2 Reactions
The table below shows the effect of solvent on this reaction.
The reaction is much faster in polar aprotic solvents.
Solvents and S
N
2 Reactions
This reaction was studied under two different conditions.
Reaction of hexyl bromide in a polar protic solvent required heating for 24 hours to form 76% of hexyl cyanide.
With a polar aprotic solvent dimethyl sulfoxide and the less reactive hexyl chloride at room temperature for 20 minutes yielded 91 % of hexyl cyanide.
Solvents and S
N
1 Reactions
Comparison of the rates of solvolysis of (CH
3
)
3
CCl and dielectric constant of the solvent shows faster reaction with more polar protic solvent.
The reaction is much faster in more polar protic solvents.
Solvents and S
N
1 Reactions
The transition state for formation of the carbocation and the halide anion is lowered in a more polar solvent.
When...
Primary alkyl halides undergo nucleophilic substitution: they always react by the S
N
2 mechanism.
Tertiary alkyl halides undergo nucleophilic substitution: they always react by the S
N
1 mechanism.
Secondary alkyl halides undergo nucleophilic substitution: they react by the
S
N
1 mechanism in the presence of a weak nucleophile (solvolysis).
S
N
2 mechanism in the presence of a good nucleophile.
Substitution and Elimination as
Competing Reactions
Substitution and/or Elimination
Lewis bases can react as nucleophiles or bases resulting in substitution or elimination.
Substitution and/or Elimination
Examples where both substitution and elimination products are formed:
The competition between substitution and elimination is:
Steric Effect on the S
N
2/E2 Reactions
The balance between substitution and elimination can be affected by changing from an unhindered base to a more hindered base.
Unhindered base (CH
3
CH
2
ONa): predominantly substitution.
Hindered base (CH
3
)
3
COK: predominantly elimination
Basicity and Substitution
Nucleophiles that are weak bases tend to give higher ratios of substitution. Azide (N
3
) and cyanide (CN ) are weak bases.
Nucleophiles and Tertiary Alkyl Halides
Tertiary alkyl halides are very hindered and yield mixtures of substitution and elimination by S
N
1 and E1 mechanisms with neutral nucleophiles.
Addition of stronger bases results in elimination exclusively through an E2 reaction.
Nucleophilic Substitution of
Alkyl Sulfonates
Nucleophilic Substitution of Sulfonates
Sulfonic acids are very strong acids similar to sulfuric acid.
Alkyl sulfonates (ROTs) are prepared by reaction of alcohols with a sulfonyl chloride:
Nucleophilic Substitution of Sulfonates
Sulfonates are stable anions and very weak bases because they are the conjugate bases of very strong acids.
Sulfonates are therefore excellent leaving groups.
Hydroxide (HO ) is a very poor leaving group so transforming an alcohol into a sulfonate generates a good leaving group.
Nucleophilic Substitution of Sulfonates
Alkyl sulfonates react faster than iodides.
Nucleophilic Substitution of Sulfonates
Sulfonates are better leaving groups than halides so they can be used to form alkyl halides by substitution.
Nucleophilic Substitution of Sulfonates
The formation of a tosylate does not change the configuration of the alcohol so an optically pure alcohol is transformed into an enantiopure tosylate.
Nucleophilic Substitution of Sulfonates
Secondary tosylates also undergo elimination with strong bases.
Secondary tosylates undergo substitution with weak bases.
Nucleophilic Substitution and
Retrosynthetic Analysis
Retrosynthetic Analysis
Retrosynthetic analysis works back from the target molecule. Here nucleophilic substitution reaction can form the thiol.
Leaving group X could be a tosylate made from an alcohol.
The alcohol could be made from an alkene.
Retrosynthetic Analysis to Synthesis
The full retrosynthetic analysis is:
Once the retrosynthetic analysis has been developed simply write the synthesis starting from the alkene.
Anti-Markovnikov addition of H-OH