Nucleophilic substitution of alkyl halides Reactions
advertisement

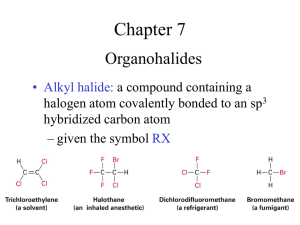
Nucleophilic substitution “NS” Lab #4 Substitution reaction: A reaction in which one atom, ion, or group is substituted for another. It is the reaction of an electron pair donor (the nucleophile, Nu) with an electron pair acceptor (the electrophile). e.g. HO:¯ + CH3CH2-Br → CH3CH2-OH + Br:¯ Good nucleophile: 1- have free pair of electrons (-ve charge ). e.g. ¯OH 2- the nucleophilicity is decrease while the electronegativity increase. e.g. ¯ OH > ¯ F , I > Br > Cl > F 3-higher molecular weight will be higher nucleophilicity. e.g. I > Br > Cl > F Leaving group: Any group that can be displaced from a carbon atom. Good leaving groups are those capable of forming stable ions or molecules upon displacement from the original molecule. SN2 mechanism (single step) there is simultaneous formation of the carbon-nucleophile bond and breaking of the carbon-leaving group bond (concerted). SN2 mechanism (single step) Energy : SN2 mechanism (single step) Energy of the molecules (average energy ) Increase the energy by heating to reach the transition state(kinetic energy). Eact= kinetic energy + average energy SN2 mechanism (single step) Rate: depend on 1. Temperature 2. Solvent 3. The concentration of the reactants R + Nu SN2 mechanism (single step) Example : SN2 mechanism (single step) Example : CH2CH2CH2CH3 + NaI Cl acetone CH2CH2CH2CH3 + NaCl I SN1 mechanism(a multi-step) In an SN1 there is loss of the leaving group generating an intermediate carbocation which then undergoes a rapid reaction with the nucleophile. SN1 mechanism(a multi-step) This pathway is multi-step process: step 1: slow loss of the leaving group, LG, to generate a carbocation intermediate (rate determining step). step 2: rapid attack of a nucleophile on the electrophilic carbocation to form a new bond SN1 mechanism Example: Step1: Step2: SN1 mechanism Energy: SN1 mechanism step1: enough energy must be supplied to break the c-x bond (Eact) & give the carbocation (intermediate). step2: need lower Eact SN1 mechanism Rate : Depends on 1- the concentration of the reactant R 2-temperature 3-solvent SN1 mechanism Reactivity order of -R : X X (CH3)3C- > (CH3)2CH- > CH3CH2- > CH33° 2° 1° Stability: the key step is the loss of the leaving group to form the intermediate carbocation. The more stable the carbocation is, the faster the SN1 reaction will be. Which substitution mechanism might operate? SN1 SN2 1-nucleophile Nucleophile strength is unimportant. Strong nucleophile is required 2-substrate 3° > 2° 1° > 2° 3-steps Multi-step 4- Formation of intermediate carbocation not 1° not 3° Single step √ X Nucleophilic substitution of alkyl halides Reactions : SN1: X= halides (Cl , Br, I ) R= CH3CH2O- = ---> Leaving group ---> electrophile (e acceptor) ---> nucleophile (e donor) Nucleophilic substitution of alkyl halides Procedure (SN1 reaction): 1- label 5 clean, dry test tubes from 1to5. 2-add 3drops of one of the following halides in test tube and immediately stopper with each addition: (1) 2-bromobutane. (2) 1-chlorobutane. (3) 2-chloro-2-methylpropane. (4) 2-bromo-2-methylpropane. (5) bromobenzene. Nucleophilic substitution of alkyl halides 3-in each test tube add 1ml of (1% AgNo3 in ethanol solution) then immediately stopper the test tube. 4-mix thoroughly and record the times for each reaction to form a precipitate or cloudiness. 5-after 5 minutes, place any test tubes that do not contain a precipitate in 100°C water bath, after about 1 minute of heating, cool the test tubes to room temperature and note whether a reaction has occurred. NOTE: consider the alkyl halide to be unreactive if no turbidity or precipitate appears after 10 min Nucleophilic substitution of alkyl halides Test tube no. Alkyl halide 1 2-bromobutane 2 1-chlorobutane 3 2-chloro-2-methylpropane 4 2-bromo-2-methylpropane 5 Bromobenzene Time Chemical reaction THANK YOU