pptx
advertisement

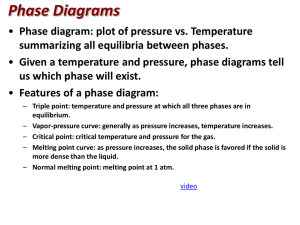
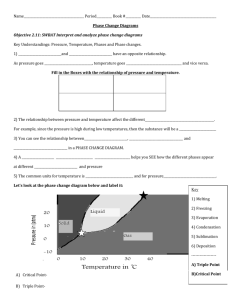
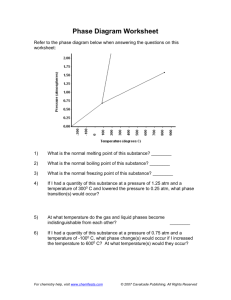
Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, Chemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 e CHEM1002 [Part 2] A/Prof Adam Bridgeman (Series 1) Dr Feike Dijkstra (Series 2) Weeks 8 – 13 Office Hours: Room: e-mail: e-mail: Monday 2-3, Friday 1-2 543a adam.bridgeman@sydney.edu.au feike.dijkstra@sydney.edu.au Slide 2/21 e Physical States and Phase Diagrams Lecture 5: • Periodic Trends Lecture 6: • Physical States: Gases, Liquids and Solids • Phase Changes • Phase Diagrams • Supercritical Fluids • Blackman, Chapter 7, Sections 7.1 and 7.3 Slide 3/21 e • • • • • Phase Changes: ΔpcH As heat is added to a solid, the molecules vibrate more and more, until.... The solid melts - an endothermic change as bonds are broken As heat is added to the liquid, the molecules move more and more, until.... The liquid boils - an endothermic change as bonds are broken As heat is added to the gas, the molecules move faster and faster Slide 4/21 e Normal Melting and Boiling Points • Vapour pressure: pressure exerted by vapour in equilibrium with its solid or liquid at stated temperature. • Normal melting point: melting point when pressure equals 1 atm (101.3 kPa). • Normal boiling point: temperature where vapour pressure of liquid equals 1 atm. Slide 5/21 e Phase Diagrams • Give temperatures and pressures where the different phases are stable. • In a one-component system, there 3 phases (solid, liquid, gas): 3 two-phase equilibria liquid-gas, solid-liquid, solid-gas 1 three-phase equilibrium all three phases co-exist: triple point Slide 6/21 e Construction of a Phase Diagram I • Plot vapour pressure of liquid as function of T (unique pressure where both phases exist at given T – co- existence curve of liquid and gas): Pressure vapour pressure = external pressure liquid gas Temperature Slide 7/21 e Construction of a Phase Diagram II Plot melting point of solid as function of pressure (co-existence curve of liquid & solid): Pressure • solid liquid Temperature Slide 8/21 Construction of a Phase Diagram III • Plot vapour pressure between solid and gas (unique pressure where both phases exist at given T – co-existence curve of solid & gas): Pressure e solid gas Temperature • Putting these all together gives phases at different P, T Slide 9/21 e Construction of a Phase Diagram Slide 10/21 e Phase Diagram for CO2 solid 1 atm liquid • At 298 K and 1 atm, CO2 is a gas decreasing the temperature, leads to deposition to solid: increasing the pressure leads to condensation to liquid sp = 195 K gas 298 K Slide 11/21 e Phase Diagram for CO2 solid liquid gas • On solid /liquid boundary, increasing pressure leads to formation of more solid • Solid is more dense than the liquid. Slide 12/21 e Phase Diagram for H2O solid liquid 273.15 K 1 atm 373.15 K gas • Starting at 1 atm and warming from T < 273 K, ice melts at 273.15 K (normal melting point) water boils at 373.15 K (normal boiling point) Slide 13/21 e Phase Diagram for H2O solid liquid gas • Triple point: three phases co-exist only at single P,T : the Kelvin scale defined is by 273.16 K = temperature at which water is at triple point • Note: Ttriple point is slightly higher than Tnormal melting Slide 14/21 e The Unusual Properties of Water • The solid-liquid slope for H2O is negative: ice melts if the pressure is increased at fixed temperature Slide 15/21 e Critical Temperature and Pressure • T & P at which boundary between liquid and vapour disappears. • If sealed tube of CO2 liquid and vapour heated, liquid-vapour phase boundary disappears at 31 °C and 73 atm: vapour T > 31 °C supercritial fluid liquid no phase boundary: cannot say that it is either liquid or vapour Slide 16/21 e Supercritical Fluids Supercritical fluids: substance above critical temperature (Tc). Can behave as solvents dissolving a wide range of substances. New industrial reaction medium. Used in dry-cleaning and food production (e.g. caffeine extraction). Slide 17/21 x Practice Examples A. B. C. D. E. (i) only (ii) only (iii) only (i) and (ii) (i) and (iii) Pressure 1. Which of the following statements concerning the phase diagram below is/are correct? (i). Moving from point A to B results in a phase transition from solid to liquid. (ii).Point D lies at the critical point. (iii).At point C, liquid and gas phases coexist in equilibrium. B A C D Temperature Slide 18/21 x Practice Examples 2. The gas methane, CH4, has a critical point at –82 °C and 46 atm. Can methane be liquefied at 25 °C? Explain your answer. (2008-J-3) 3. Which of the following gases can be liquefied at 25 °C? Gas Critical point CH3Cl 144 °C, 66 atm SO2 158 °C, 78 atm CH4 –82 °C, 46 atm (A) SO2 only (B) CH4 only (C) CH3Cl and SO2 (D) all of them (E) none of them Slide 19/21 x Practice Examples 4. You may recall from a lecture demonstration or your laboratory work that solid CO2 sublimes under ambient conditions while ice melts. Define the terms sublimation and melting. 5. What is a triple point (e.g. in the phase diagram of CO2 or H2O)? 6. What does the different behaviour of ice and solid CO2 indicate about the relative positions of their respective triple points? 2007-N-6 Slide 20/21 e Summary: Phase Diagrams Learning Outcomes - you should now be able to: • • • • Complete the worksheet Identify and explain the types of intermolecular and intramolecular forces Define and give two examples of allotropes Answer Review Problems 6.28-6.32 and 7.5-7.7 in Blackman Next lecture: • 2 component phase diagrams and entropy Slide 21/21