Exam 2 Review - Iowa State University
advertisement
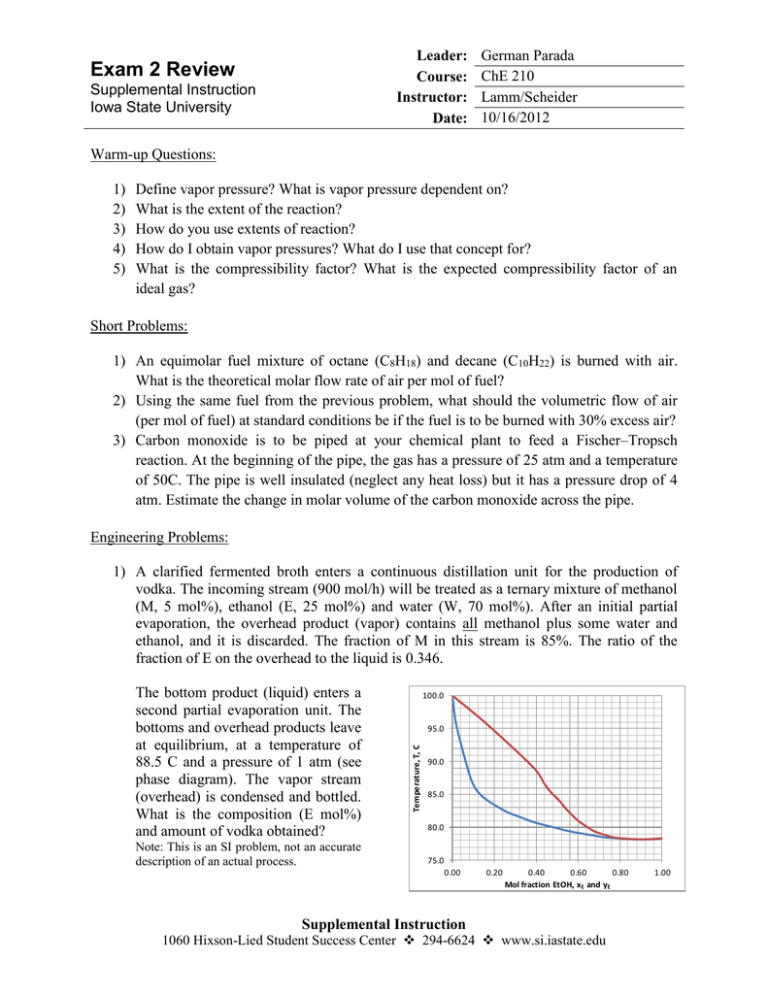
Leader: Course: Instructor: Date: Exam 2 Review Supplemental Instruction Iowa State University German Parada ChE 210 Lamm/Scheider 10/16/2012 Warm-up Questions: 1) 2) 3) 4) 5) Define vapor pressure? What is vapor pressure dependent on? What is the extent of the reaction? How do you use extents of reaction? How do I obtain vapor pressures? What do I use that concept for? What is the compressibility factor? What is the expected compressibility factor of an ideal gas? Short Problems: 1) An equimolar fuel mixture of octane (C8H18) and decane (C10H22) is burned with air. What is the theoretical molar flow rate of air per mol of fuel? 2) Using the same fuel from the previous problem, what should the volumetric flow of air (per mol of fuel) at standard conditions be if the fuel is to be burned with 30% excess air? 3) Carbon monoxide is to be piped at your chemical plant to feed a Fischer–Tropsch reaction. At the beginning of the pipe, the gas has a pressure of 25 atm and a temperature of 50C. The pipe is well insulated (neglect any heat loss) but it has a pressure drop of 4 atm. Estimate the change in molar volume of the carbon monoxide across the pipe. Engineering Problems: 1) A clarified fermented broth enters a continuous distillation unit for the production of vodka. The incoming stream (900 mol/h) will be treated as a ternary mixture of methanol (M, 5 mol%), ethanol (E, 25 mol%) and water (W, 70 mol%). After an initial partial evaporation, the overhead product (vapor) contains all methanol plus some water and ethanol, and it is discarded. The fraction of M in this stream is 85%. The ratio of the fraction of E on the overhead to the liquid is 0.346. Note: This is an SI problem, not an accurate description of an actual process. 100.0 95.0 Temperature, T, C The bottom product (liquid) enters a second partial evaporation unit. The bottoms and overhead products leave at equilibrium, at a temperature of 88.5 C and a pressure of 1 atm (see phase diagram). The vapor stream (overhead) is condensed and bottled. What is the composition (E mol%) and amount of vodka obtained? 90.0 85.0 80.0 75.0 0.00 0.20 0.40 0.60 0.80 Mol fraction EtOH, xE and yE Supplemental Instruction 1060 Hixson-Lied Student Success Center 294-6624 www.si.iastate.edu 1.00 a – Complete labeling the flowchart. b – Carry out a DoF analysis for both units and the overall process. Which system can you solve for first? c – Write the equations and indicate how you would solve for the quantities asked. 2) A stream of pure gaseous propylene (P -- CH3CHCH2) is fed to a polymerization reactor at a temperature of 130 C and pressure of 25 bar. The measured flow rate is 6615 gal/min. At the reactor, polypropylene (PP) is produced as followed: 1100 P PP1100 (polym1) 1200 P PP1200 (polym2) The selectivity of PP1200 to PP1100 is 7.5 and the propylene conversion is 0.60. The polymers form pellets that are separated from the unreacted polypropylene stream immediately after the reactor. This unreacted stream is then divided; 95% of it is recycled back to the process. This recycle stream is mixed with a certain amount of fresh propylene (at STP) before a compressor, and this constitutes the reactor feed stream. How many moles of polymer 2 are obtained? What is the volumetric flow rate of fresh propylene required by this process? A flow chart has been provided. a – Complete a DoF of the reactor, and indicate of the system can be solved. b – Write the equations of the reactor, indicating how you would get the amount of polymers produced. c – Show how to get the volumetric flow rate of fresh propylene, but don’t solve for it.