The Periodic Table

The Periodic Table
Periodic Table
Dmitri Mendeleev
(1834-1907)
"We could live at the present day without a Plato, but a double number of Newtons is required to discover the secrets of nature, and to bring life into harmony with the laws of nature."
Modern Periodic Table
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
H: 1s
1
1s n = 1 l = 0 m l
= 0
2s 2p n = 2 l = 0 m l
= 0 m l
= -1 n = 2 l = 0 m l
= 0 m l
= 1
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
He: 1s
2
1s n = 1 l = 0 m l
= 0
2s 2p n = 2 l = 0 m l
= 0 m l
= -1 n = 2 l = 0 m l
= 0 m l
= 1
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
1s
Li: 1s
2
2s
1 n = 1 l = 0 m l
= 0
2s 2p n = 2 l = 0 m l
= 0 m l
= -1 n = 2 l = 0 m l
= 0 m l
= 1
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
1s
Be: 1s
2
2s
2 n = 1 l = 0 m l
= 0
2s 2p n = 2 l = 0 m l
= 0 m l
= -1 n = 2 l = 0 m l
= 0 m l
= 1
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
1s
B: 1s
2
2s
2
2p
1
‘core’ closed shell
2s 2p open shell: valence electrons
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
1s 2s 2p
C: 1s
2
2s
2
2p
2
Hund’s rule: maximum number of unpaired electrons is the lowest energy arrangement.
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
N: 1s
2
2s
2
2p
3
O: 1s
2
2s
2
2p
4
1s 2s 2p
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
F: 1s
2
2s
2
2p
5
Ne: 1s
2
2s
2
2p
6
1s 2s 2p
s- and p-orbitals
‘Aufbau’ Principle: filling orbitals
Na: 1s 2 2s 2 2p 6 3s 1 or [Ne]3s 1
Mg: 1s 2 2s 2 2p 6 3s 2 or [Ne]3s 2
P: [Ne]3s 2 3p 3
Ar: [Ne]3s 2 3p 6
4s
3s
E
2s
3p d-orbitals
3d
2p
1s
Due to deeper penetration of s-orbitals, 4s lies lower in energy than 3d
d-orbitals
K: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 or [Ar]4s 1
Ca: [Ar]4s 2
Sc: [Ar]4s 2 3d 1
V: [Ar]4s 2 3d 3
Cr: [Ar]4s 1 3d 5
Co: [Ar]4s 2 3d 7
Cu: [Ar]4s 1 3d 10
Zn: [Ar]4s 2 3d 10
Ga: [Ar]4s 2 3d 10 4p 1
Kr: [Ar]4s 2 3d 10 4p 6
‘s’-groups
Beyond the d-orbitals
‘p’-groups d-transition elements lanthanides actinides f-transition elements
Aufbau rules
1. Within a shell ( n ) the filling order is s>p>d>f
2. Within a subshell ( l ), lowest energy arrangement has the highest number of unpaired spin (Hund’s rule)
3. The (n+1)s orbitals always fill before the nd orbitals
4. After lanthanum ([Xe]6s 2 5d 1 ), the 4f orbitals are filled
5. After actinium ([Rn]7s 2 6d 1 ), the 5f orbitals are filled
Filled subshells accommodate: s: p:
2 electrons
6 electrons d: f:
10 electrons
14 electrons
Electron configuration
Give the electron configuration of Zirconium and Tellurium.
Identify the period and the group of the element
Zirconium is in period 5 and is the 2nd element in the d-transition element group.
Zr: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 2 or [Kr]5s 2 4d 2
Tellurium is in period 5 and is the 4th element in the ‘p’- group.
Te: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 4 or [Kr]5s 2 4d 10 5p 4
Exotic elements
Elements with atomic numbers higher than 92 (Uranium) typically don’t exist in nature and have to be made by nuclear synthesis
The first synthesized elements were named after the planets: uranium neptunium plutonium
92
Ur
93
Np
94
Pu
99
Es
101
Md
107
Bh
110
Uun
Exotic elements
Einsteinium
Mendelevium
No name yet!
Bohrium
Lives for only 10 ms!
Barbarium?
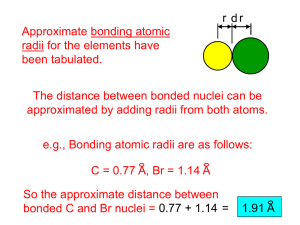
Atomic Radius
How big is an atom?
The atomic radius r is usually determined from the distances between atoms in covalent bonds.
Atomic radius decreases across a period from left to right due to increased effective nuclear charge
Atomic radius increases down a group because of the larger sizes of the orbitals with higher quantum numbers.
Atomic Radius
Atomic Radius
Atomic Radius
Covalent radius is much smaller than the anionic radius.
Atomic Radius
Arrange the following sets of atoms in order of increasing size:
Sr, Se, Ne :
Fe, P, O :
Ne(10) < Se(34) < Sr(38)
O(8) < P(15) < Fe(26)
Arrange the following sets of ions in order of increasing size:
Na + , Rb + , Li + :
Cl , F , I :
Li + (3) < Na + (11) < Rb + (37)
F (9) < Cl (17) < I (53)
Ionization Energy
Ionization energy is the energy required to remove an electron from a gaseous atom or ion : e -
+
X(g) X + (g) + e -
S(g) S + (g) + e I
1
= 999.6 kJ/mol
1st ionization energy
S + (g) S 2+ (g) + e I
2
= 2251 kJ/mol
2nd ionization energy
S 2+ (g) S 3+ (g) + e I
3
= 3361 kJ/mol
3rd ionization energy
Ionization Energy
S(g) S + (g) + e I
1
= 999.6 kJ/mol
1st ionization energy
S + (g) S 2+ (g) + e I
2
= 2251 kJ/mol
2nd ionization energy
S 2+ (g) S 3+ (g) + e I
3
= 3361 kJ/mol
3rd ionization energy
S: 1s 2 2s 2 2p 6 3s 2 3p 4
Which electrons are removed in successive ionizations?
Electrons in the outer subshells take the least amount of energy to remove (valence electrons)
It takes about 1•10 3 kJ/mol to remove successive electrons from the 3p shell of sulfur.
Ionization Energy
Ionization energies of aluminum:
Al(g) Al + (g) + e I
1
= 580 kJ/mol
1st ionization energy
Al + (g) Al 2+ (g) + e I
2
= 1815 kJ/mol
2nd ionization energy
Al 2+ (g) Al 3+ (g) + e I
3
= 2750 kJ/mol
3rd ionization energy
Al 3+ (g) Al 4+ (g) + e I
4
= 11,600 kJ/mol
4th ionization energy
Al: 1s 2 2s 2 2p 6 3s 2 3p 1
1st electron: 3p valence electron
2nd electron: 3s valence electron
3rd electron: 3s valence electron
4th electron: 2p core electron!
core electrons take much more energy to remove
Ionization Energy
Ionization Energy
First ionization energies
General trends:
Ionization energy increases across the period from left to right.
Ionization energy decreases going down a group
Ionization Energy
A closer look…..
B: 1s 2 2s 2 2p 1
O: 1s 2 2s 2 2p 4
New subshell, electron is easier to remove.
First paired electron in 2p orbital: repulsion.
Understanding a group
Atoms in a group have the same valence electron configuration and share many similarities in their chemistry.
Li
Group 1A: Alkali metals
Na K
Cs
Understanding a group
Group 1A: Alkali metals
Trends down the group reflect periodic changes in mass, volume and charge.
Periodic Table in Brief
Periodic Table Redux
Periodic Table Redux
![The electronic configuration of phosphorus is [Ne] 3s2 3p3](http://s3.studylib.net/store/data/008974852_1-8381577ce936fbfa611892c1a5f109cd-300x300.png)