Chem 11 - Periodic Trends Worksheet
advertisement

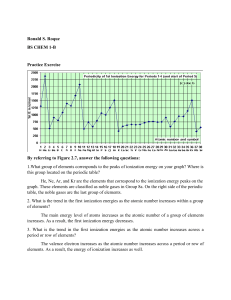
Chem 11 - Periodic Trends Worksheet Read p. 49 – 58 and answer the following questions on this worksheet. These will serve as your notes for this section. 1) How is the atomic size (radius) measured? Is there a definite border? 2) Give the trend for atomic size going down a group and across (to the right) of a period. Draw a diagram. 3) Explain briefly the reasons for the trends in atomic size. 4) Define the following: ion, cation, anion, ionization energy. Which types of elements form cations, which form anions? 5) What are the units of ionization energy? What is the first ionization energy? 6) Summarize the trends for ionization energy. Draw a diagram. 7) Explain briefly the reasons for the trends in ionization energy. 8) Define electron affinity. What are its units? What do high negative numbers, low negative numbers, and positive electron affinities mean? (bottom of p. 57). 9) Explain the general trends in electron affinities. Draw a diagram. HWK: p. 52 #7, p. 55 # 8, 9 p. 60 # 2, 3, 4