2.the nature of lipids
advertisement

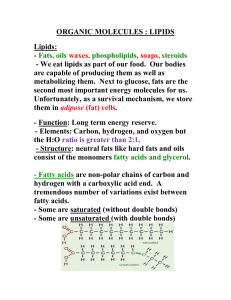
© SSER Ltd. The Nature of Lipids Lipids are a diverse collection of substances that have a range of different functions in living systems Lipids are compounds that serve both as structural and nutrient substances The lipid group includes fats and oils, waxes, steroids and phospholipids. These molecules have a low solubility in water but a high solubility in solvents such as ethanol and chloroform Fats & oils are formed from molecules of glycerol and fatty acids The Components of Fats & Oils Glycerol is a 3-carbon alcohol molecule Fatty acids are composed of hydrocarbon chains of varying length with a methyl group at one end and a carboxylic acid group at the other A Fatty Acid Carboxylic acid group Glycerol Hydrocarbon chain Methyl group Saturated & Unsaturated Fatty Acids 2 3 Saturated fatty acid 2 n 3 General formula for a saturated fatty acid Double bond Unsaturated fatty acid: less saturated with hydrogen atoms 3 Formation of a Triglyceride THREE FATTY ACID MOLECULES GLYCEROL -3H2O Condensation Reaction A TRIGLYCERIDE Ester bond Phospholipids Fatty acid Glycerol Fatty acid Phosphate hydrophobic tails hydrophilic phosphate group OR Fatty acid Glycerol Phosphate Fatty acid Phospholipids The phosphate-containing end of the phospholipid molecule is soluble in water, while the hydrophobic fatty acid tails orientate themselves in positions away from a watery medium Polar phosphate head (hydrophilic) The bipolar nature of phospholipids allows these molecules to form bilayers that form a major component of cell membranes Hydrophobic fatty acid (lipid) tails PHOSPHOLIPID BILAYER water water Cholesterol molecules are located between the tails of the phospholipid molecules where they serve to stabilise the membrane These cholesterol molecules are also classed as LIPIDS although they belong to a very different sub-group known as STEROIDS phospholipid bilayer of cell membrane cholesterol stabilising the membrane Summary Fats & oils are made up of carbon, hydrogen and oxygen atoms. The building blocks (monomers) of fats and oils are glycerol & fatty acid molecules. Fats & oils are TRIGLYCERIDES. Three fatty acid molecules bond to each glycerol molecule by CONDENSATION REACTIONS. The bonds formed from these condensation reactions are called ESTER BONDS. Fats & oils are chemically similar but physically different. Fats are solid at room temperature whereas oils are liquid. Monoglycerides & diglycerides also form when glycerol and fatty acids bond by condensation reactions. Monoglycerides form when only ONE FATTY ACID bonds with a glycerol molecule. Diglycerides form when TWO FATTY ACIDS bond with a glycerol molecule. Phospholipids are DIGLYCERIDES. Phospholipids form when TWO FATTY ACIDS and a PHOSPHATE group bond to a glycerol molecule. The phosphate end of the molecule is hydrophilic (water-loving) and the two fatty acids tails are hydrophobic (water-hating). Phospholipids are a major structural component of cell membranes. Steroids such as cholesterol, oestrogen and progesterone also belong to the class lipids.