Moles - Fulton County Schools
advertisement

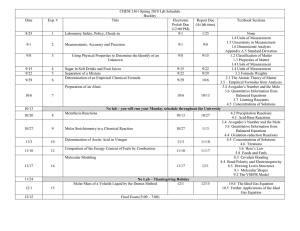
Department: ___Science_______________ Teacher: ___Davis_________________ Course: ____Honors Chemistry______________ Topic / Theme: Chemical Composition of Compounds and Molar Calculations______________________ Duration:_____~ 2 weeks_____________________ Essential Question: Key Questions: Essential Vocabulary: What is the relationship between mass, moles, particles and volume of a substance? What is the mole ? How do you determine the molar mass of an element? How do you determine the molar mass of a compound? How do you convert between grams, moles, number of atoms, number of molecules and the number of formula units for elements and compounds? How are your calculations rounded to the correct number of significant figures? Given the percent composition of a compound (including hydrates), how do you determine its empirical formula? Given the percent composition of a compound (including hydrates) and its molar mass, how do you determine the molecular formula? How do you determine the percent composition of a compound if given the chemical formula? How are empirical formulas for compounds determined experimentally? Mole, Avogadro’s number, hydrate, molar mass, Empirical formula, molecular formula, percent composition, molar volume, standard temperature and pressure, atom, molecule, formula unit Standards (List the primary standard and the key verbs from the elements) Higher-Order Student Engagement (Specific activities/tasks that will actively engage students in higher order learning) SC2 Students will relate how the Law of Conservation of Matter is used to determine the chemical composition of compounds and chemical reactions c. Apply concepts of the mole and Avogadro’s number to conceptualize and calculate: Empirical / molecular formulas Mass, moles, molecules and formula units relations Molar volumes of gases Assessment of Learning Goals : Formative and Summative Call on specific students with content specific questions every day. Nightly Homework Problems. Unit Review Homework/Study Guide. Laboratory Activities. Unit Test (honors chemistry tasks require students to apply knowledge to solve problems) Laboratories (How do you know if your students have learned?) Call on specific students with content specific questions every day. Nightly Homework Problems. Unit Review Homework/Study Guide. Laboratory Activities. Unit Test Chalk mole conversion lab Empirical formula determination of magnesium oxide Empirical formula determination of hydrated copper (II) sulfate We empower students to discover, think, and succeed. GPS Standards (Optional) SC2 Students will relate how the Law of Conservation of Matter is used to determine the chemical composition of compounds and chemical reactions c. Apply concepts of the mole and Avogadro’s number to conceptualize and calculate: Empirical / molecular formulas Mass, moles, molecules and formula units relations Molar volumes of gases We empower students to discover, think, and succeed.