Review of the Gas Laws
advertisement

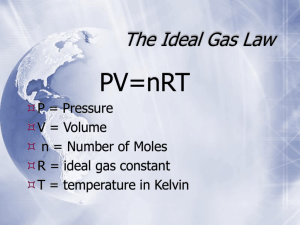
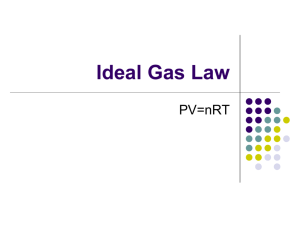
Review of Gases and the Gas Laws PV = nRT Kinetic Molecular Theory Postulates: • A gas consists of a collection of small particles traveling in straight-line motion and obeying Newton's Laws. • The molecules in a gas occupy no volume (that is, they are points). • Collisions between molecules are perfectly elastic (that is, no energy is gained or lost during the collision). • There are no attractive or repulsive forces between the molecules. • The average kinetic energy of a molecule is 3kT/2. (T is the absolute temperature and k is the Boltzmann constant.) PV = nRT • • • • • • Boyle’s Law (isothermal & fixed amount) Charles’s Law (isobaric & fixed amount) Avogadro’s Law (isothermal & isobaric) Ideal Gas Law (isochoric & fixed amount) Ideal Gas Law (isothermal & isochoric) Ideal Gas Law (isobaric & isochoric) Boyle’s Law PV nRT or P1V1 P2V2 L atm PV (1mol) 0.0821 (273K ) K mol P V 22.4 L atm P V 22.4 L atm • Pressure and volume are inversely proportional. • As pressure increases, volume decreases. • If pressure increases by 2x, volume cuts in half. Charles’s Law V nR V1 V2 or T P T1 T L atm (1 mole) 0.0821 V K mol T 1 atm • Temperature and volume are directly proportional. • As temperature increases, volume also increases. • If temperature increases by 2x, volume also doubles. • Temperature must be measured in Kelvin. Avogadro’s Law V RT n P L atm 0.0821 (273K ) V K mol n (1atm) V L 22.4 n mol • Moles of gas and volume are directly proportional. • As the number of moles increases, the volume also increases. • If the number of moles increases by 2x, the volume also doubles. PV = nRT P V T n R = = = = = pressure volume temperature (Kelvin) number of moles gas constant Standard Temperature and Pressure (STP) T = 0 oC or 273 K P = 1 atm = 101.3 kPa = 760 mm Hg 1 mol = 22.4 L @ STP Solve for constant (R) PV nT Recall: 1 atm = 101.3 kPa Substitute values: (1 atm) (22.4 L) (1 mole)(273 K) = R R = 0.0821 atm L / mol K R = 0.0821 atm L mol K or (101.3 kPa) ( 1 atm) R = 8.31 kPa L / mol K = 8.31 kPa L mol K Ideal Gas Law – P & n P RT n V L atm 0.0821 (273K ) P K mol n 22.4 L P atm 1 n mol • Moles of gas and pressure are directly proportional. • As the moles of gas increase, the pressure also increases. • If the number of moles of gas increases by 2x, the pressure also doubles. Ideal Gas Law – n vs T nT PV R nT (1atm)( 22.4 L) L atm 0.0821 K mol n T 273K mol • Moles of gas and temperature are inversely proportional. • As the number of moles of gas increase, the temperature decreases. Units of Pressure • 1 atm = 760 torr = 760 mmHg • 1 atm = 101.325 kPa • 1 bar = 105 Pa = 100 kPa • 1 Pa = 1N 2 m Dalton’s Law of Partial Pressures Pressure is additive. Write each pressure as, PT Pa Pb nRT P V nT RT na RT nb RT V V V Continue Dalton’s Law of Partial Pressures nT RT na RT nb RT V V V Multiply through by V (the combined volume of the gases) and divide by R T. nT na nb Moles are indeed additive. Mole Fraction & Partial Pressure na Xa nT and nb Xb nT na nb na nb Xa Xb nT nT nT Therefore, Xa Xb 1 or nT nT Collecting Gas Over Water Pgas P PH 2O Gas Law Problem #1 2 HCOONa + H2SO4 2 CO + 2 H2O + Na2SO4 A 0.964 gram sample of a mixture of sodium formate and sodium chloride is analyzed by adding sulfuric acid. The equation for the reaction for sodium formate with sulfuric acid is shown above. The carbon monoxide formed measures 242 milliliters when collected over water at 752 torr and 22.0°C. Calculate the percentage of sodium formate in the original mixture. Gas Law Problem #2 A 6.19 gram sample of PCl5 is placed in an evacuated 2.00 liter flask and is completely vaporized at 252°C. (a)Calculate the pressure ion the flask if no chemical reaction were to occur. (b) Actually at 252°C the PCl5 is partially dissociated according to the following equation: PCl5(g) PCl3(g) + Cl2(g) The observed pressure is found to be 1.00 atmosphere. In view of this observation, calculate the partial pressure of PCl5 and PCl3 in the flask at 252°C. Gas Problem #3 A mixture of H2(g), O2(g), and 2 millilitres of H2O(l) is present in a 0.500 litre rigid container at 25°C. The number of moles of H2 and the number of moles of O2 are equal. The total pressure is 1,146 millimetres mercury. (The equilibrium vapor pressure of pure water at 25°C is 24 millimetres mercury.) The mixture is sparked, and H2 and O2 react until one reactant is completely consumed. (a) Identify the reactant remaining and calculate the number of moles of the reactant remaining. (b) Calculate the total pressure in the container at the conclusion of the reaction if the final temperature is 90°C. (The equilibrium vapor pressure of water at 90°C is 526 millimetres mercury.) (c) Calculate the number of moles of water present as vapor in the container at 90°C. Answer: (a) 2 H2 + O 2 2 H2 O mol H2 = mol O2 initially, but 2 mole H2 react for every 1 mol of O2, therefore, O2 is left. PT = PH2 + PO2 + PH2O 1146 mm Hg = PH2 + PO2 + 24 mm Hg PH2 + PO2 = 1122 mm Hg 1122 mm Hg / 4 = PO2 left (1/2 of initial PO2 which is 1/2 total) PO2 = 280.5 mm Hg (b) 28 0 . 5 mmHg 29 8 K PO 2 36 3 K ; P O 34 2 mmHg 2 PT = PO2 + PH2O = (342 + 526)mm Hg = 868 mm Hg (c) n PV RT 52 6 ( 7 6 0 atm )( 0 . 50 0 L ) 0 . 08 21 L a t m m ol K ( 363 K ) 0 . 011 6 m o l What Makes a Gas Ideal? The ideal gas law, PV = nRT, relates variables that describe a sample of an ideal gas. To be an ideal gas: • Size of gas molecules is negligible relative to distances between adjacent molecules. Most of volume occupied by a gas is empty space. • Gas molecules are in constant random motion and each molecule continues to travel in a straight line unless it collides with another molecule or with wall of container. • Gas molecules do not attract each other and all collisions are elastic. • Average kinetic energy of molecules is proportional to absolute temperature. What makes a gas “Real”? Real gases: • follow ideal gas equation only at low pressures and high temperature • Boyle’s Law doesn’t hold at high pressures (PV ≠ C) • Charles’ Law doesn’t hold at low temperatures (V/T ≠ C). • Deviations of real gases and from the Ideal Gas Equation, worsen as temperature decreases and pressure increases. These deviations are revealed in the compressibility factor (z) of the gas, where: z = PV/nRT For ideal gases, z=1 under all conditions. For real gases, z ≠ 1.The farther away from 1 that z gets, the more poorly the ideal gas equation is being followed. Comparison of Real vs. Ideal Gases Ideal Gases Real Gases