Ideal Gas Law
advertisement

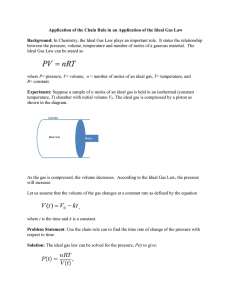
Ideal Gas Law PV=nRT Kinetic Molecular Theory 1. Gases have low density 2. Gases have elastic collisions 3. Gases have continuous random motion. 4. Gases have no attraction or repulsion 5. Kinetic energy is proportional to temperature. Properties of Gases Pressure (P) Volume (V) The amount of space occupied by a gas Temperature (T) Force of gas particles hitting the sides of their container The Kinetic Energy of gas molecules Mols (n) The amount of gas molecules in a certain mass Ideal Gas Law “the mathematical relationship among pressure, volume, temperature, and the number of moles of a gas” What are the observable relationships between these properties? Pressure and Temperature Pressure and Volume Pressure and moles Volume and Temperature Volume and moles Temperature and moles Partial Gas Laws Boyle’s Law V=k/P V : 1/P Charle’s Law V = kT V:T Gay-Lussac’s Law P=kT P:T Avogadro’s Law Standard Molar volume V:n Ideal Gas Law V : 1/P V:T V:n V : nT/P V = constant * nT/P PV = constant * nT Constant = R PV = nRT Units: (for this class) V must be in Liters P must be in atm T must be in Kelvin n is always mols Ideal Gas Constant = R 0.0821(L*atm) / (mol * K) Ideal Gas Law Example How many moles of gas are in 4.6 L of gas at 56kPa and 34oC? Convert all units to the right units Volume: 4.6 L is in correct units Pressure: 56 kPa x (1atm/101.3kPa) = 0.55 atm Temperature: 34oC + 273 = 307K Write Ideal Gas Law Equation PV = nRT Ideal Gas Law Example How many moles of gas are in 4.6 L of gas at 56Pa and 34oC? PV = nRT Solve for unknown n = PV / (RT) Plug in numbers n =(0.55 atm * 4.6 L) / (0.0821 * 307K)= 0.10mols Simulation http://www.chem.ufl.edu/~itl/2045/MH_sims/g as_sim.html