Gasses - My CCSD
advertisement
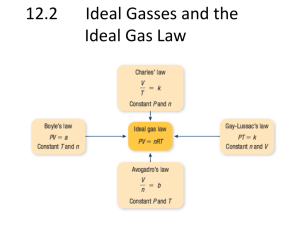
Gasses Kinetic molecular theory Pressure Introduction • Earth’s atmosphere is a gaseous solution composed of mostly nitrogen and oxygen • The atmosphere is important for life on earth – Provides a medium for many chemical reactions with waste products – Shields the Earth from harmful radiation – Retains heat on earth’s surface • The behavior of gas particles contributes to their important properties States of Matter • Atoms/Molecules in all states of matter have attractive forces – Strongest in solids, weakest in gasses – Affected by changes in temperature • Kinetic energy (KE) = energy of motion • Temperature = a measurement of kinetic energy Solids Definite shape X Definite volume X X Gases X X Takes the shape of its container Cannot be compressed Liquids X X Kinetic Molecular Theory • Gasses consist of tiny particles • The particles are so small (compared between the distances between them) that the individual particles have nearly no volume • The particles are in constant random motion, colliding with the walls of the container. These collisions cause the pressure exerted by the gas. • The particles are assumed not to attract or repel each other. • The average kinetic energy of the gas particles is proportional to the Kelvin temperature of the gas. Pressure • Gas fills any container uniformly – It is evenly spread out • Gasses exert pressure on their surroundings – Example: when you blow up a balloon, the gasses inside push against the sides and keep it firm. • A Barometer measures atmospheric pressure – Invented by Evengelista Torricelli in 1643 • Atmospheric pressure results from the air being pulled by gravity – Varies with altitude – Changes with weather conditions Units of Pressure • The units for pressure are based on the height of the mercury column (in millimeters) that the gas pressure can support – mm Hg (millimeters of mercury) is also called torr • Standard atmospheres (abbreviated atm) are also used. 1 standard atm = 1.000 atm = 760.0 mm Hg = 760.0 torr • The SI unit for pressure is the pascal (Pa) 1 atm = 101,325 Pa • Engineers use pounds per square inch (psi) 1.000 atm = 14.69 psi Pressure Unit Conversions Convert the following to atmospheres: 1. 2. 3. 109.2 kPa (1 kPa = 1000 Pa) 781 torr 15.2 mm Hg Convert the following into units of mm Hg: 4. 5. 6. 9.75 psi 121.4 kPa 1.14 atm Convert the following into kilopascals (kPa) 7. 8. 9. 10. 105,390 Pa 764 mm Hg 1.29 atm 697 torr