KMT and Properties of Gases
advertisement
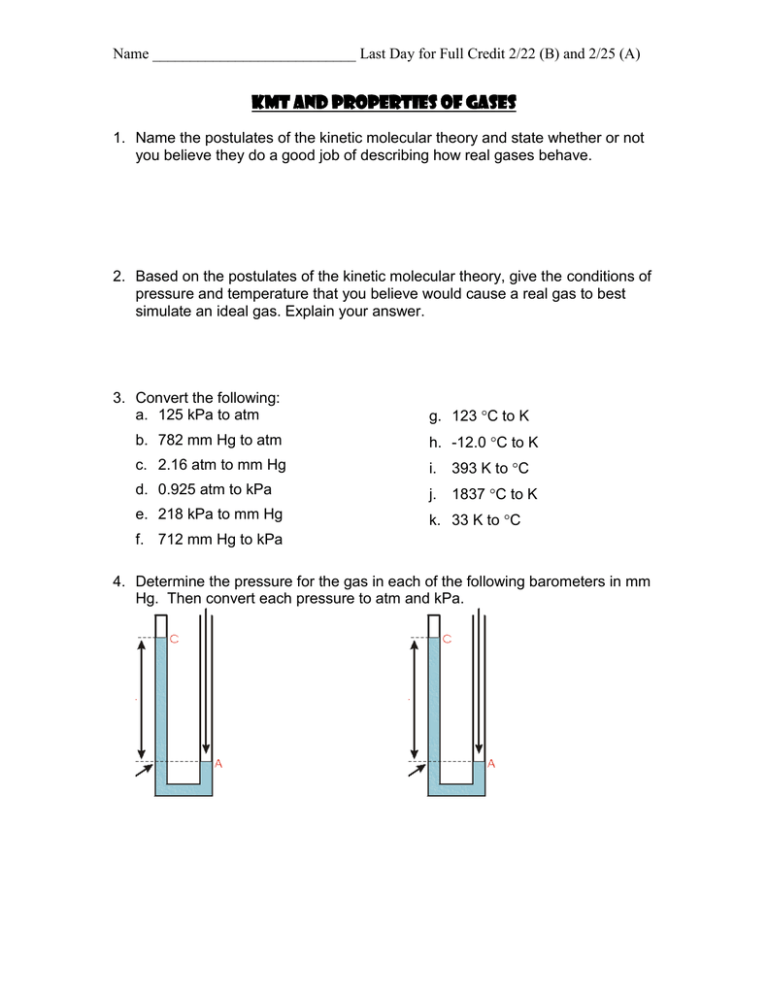
Name ___________________________ Last Day for Full Credit 2/22 (B) and 2/25 (A) KMT and Properties of Gases 1. Name the postulates of the kinetic molecular theory and state whether or not you believe they do a good job of describing how real gases behave. 2. Based on the postulates of the kinetic molecular theory, give the conditions of pressure and temperature that you believe would cause a real gas to best simulate an ideal gas. Explain your answer. 3. Convert the following: a. 125 kPa to atm g. 123 C to K b. 782 mm Hg to atm h. -12.0 C to K c. 2.16 atm to mm Hg i. 393 K to C d. 0.925 atm to kPa j. 1837 C to K e. 218 kPa to mm Hg k. 33 K to C f. 712 mm Hg to kPa 4. Determine the pressure for the gas in each of the following barometers in mm Hg. Then convert each pressure to atm and kPa. Name ___________________________ Last Day for Full Credit 2/22 (B) and 2/25 (A) 5. Write whether the following statements describe a real gas or an ideal gas or both. a. The gas liquefies at high pressures. _____________ b. The gas particles have no attraction for each other ____________ c. The gas particles have no volume _____________ d. The gas particles take up a small amount of space __________ e. The gas particles bounce off each other and the walls of the container ______________ f. The gas particles collide and transfer, but do not lose energy __________ 6. Explain what happens on the molecular level when the temperature of a gas sample increases. 7. Explain what happens on the molecular level when the pressure of a gas sample increases.











