Introduction to Earth Science - wbm-earth
advertisement
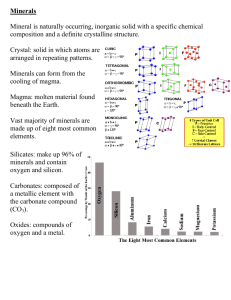
Minerals of Earth’s Crust Chapter 3 Earth Science Chapter 3 1 Mineral • A naturally occurring, inorganic solid that has a crystal structure and a definite chemical composition. Earth Science Chapter 3 2 Mineral • Must occur naturally • Formed by processes in natural world • Not manufactured (made by man) Earth Science Chapter 3 3 Mineral • Must be inorganic • Not made of living things or the remains of living things • Nonexample: coal • Comes from the remains of plants and animals Earth Science Chapter 3 4 Mineral • Must be a solid • Definite (unchanging) shape and definite volume • Particles are tightly packed Earth Science Chapter 3 5 Mineral • Must have a crystal structure • Has flat sides that meet at sharp edges and corners • Atoms arranged in regular, repeating pattern Earth Science Chapter 3 6 Mineral • Must have a definite chemical composition • Always contains certain elements in the same proportions • Example: Quartz has one atom of silicon for every two atoms of oxygen • Pure, solid elements (mostly metals) are also minerals Earth Science Chapter 3 7 Physical properties of minerals • Result of chemical composition and crystalline structure • Useful for identifying minerals • Many can be seen with the eye • Others need tests and special equipment Earth Science Chapter 3 8 Color • Easy to observe • Not very useful • Many have similar colors • Small amounts of impurities can dramatically change colors • Ruby and sapphire are both types of corundum • Weathered surfaces may hide color Earth Science Chapter 3 9 Streak • Color of mineral in powdered form • More reliable than just color • Tested by rubbing across a streak plate of unglazed ceramic tile • Streak color may be different than mineral color • Even though the color of a mineral may vary, its streak color is always the same • Minerals that are harder than streak plate leave no streak Earth Science Chapter 3 10 Luster • Light that is reflected from a mineral’s surface • Metallic – like polished metal • Or nonmetallic • • • • • • • Glassy Waxy Greasy Pearly Submetallic or dull Silky Earthy Earth Science Chapter 3 11 Discuss • Define “mineral” in your own words. • Amber is a precious material used in jewelry. It forms when the resin of pine trees hardens into stone. Is amber a mineral? Explain. • What does the phrase “definite chemical composition” mean? Earth Science Chapter 3 12 Density • The amount of mass in a given space • Mass per unit volume • You can compare the densities of two minerals by lifting two samples that are the same size and seeing which is heavier • Calculate density by taking mass divided by volume • Depends on the kinds of atoms in a mineral and how closely they are packed. Earth Science Chapter 3 13 Hardness • Measure of the ability to resist scratching • Determined by the strength of the bonds between atoms Earth Science Chapter 3 14 Mohs Hardness Scale • Standard scale for mineral hardness • To test an unknown mineral you must determine the hardest mineral on the scale it can scratch. • If neither of two minerals scratches the other, they have the same hardness Earth Science Chapter 3 15 Crystal Structure • Six basic shapes • A certain mineral always has the same basic shape Earth Science Chapter 3 16 Cleavage • Tendency to split along specific planes of weakness to form smooth, flat surfaces Earth Science Chapter 3 17 Fracture • Tendency to break unevenly into pieces that have curved or irregular surfaces • Described by how it looks when it breaks • • • • Shell-shaped fracture Hackly fracture (jagged points) Earthy fracture (crumbly) Uneven fracture (rough surface) Earth Science Chapter 3 18 Special properties • Fluorescence – the ability to glow under ultraviolet light • Phosphorescence – the ability to keep glowing after the ultraviolet light is turned off • Chemical reactivity Earth Science Chapter 3 19 Special properties • Asterism – a six-sided star appears in reflected light Earth Science Chapter 3 20 Special properties • Double refraction • Light bending through the mineral produces a double image • Magnetism • Radioactivity • Unstable • Decay over time releasing particles and energy Earth Science Chapter 3 21 Discuss • Name eight properties that are used to identify minerals • Compare and contrast fracture and cleavage. • Graphite is made of carbon atoms arranged in thin sheets. The sheets are weakly held together. Predict whether graphite will break apart with fracture or cleavage. Explain. Earth Science Chapter 3 22 Crystallization • How atoms in a crystal structure get arranged in that structure Earth Science Chapter 3 23 Minerals from magma and lava • Magma – molten rock beneath Earth’s surface • Lava – what magma becomes when it reaches the surface • Both form crystals as they cool and solidify Earth Science Chapter 3 24 Cooling rates • Magma far underground cools slowly • Large crystals, especially if undisturbed • Lava and magma close to the surface cool more quickly • Smaller crystals Earth Science Chapter 3 25 Minerals from solutions • Solution – one substance dissolved in another • Minerals dissolved in water • Crystallize when they leave the solution Earth Science Chapter 3 26 Evaporation • Minerals formed at the edges of bodies of water • Minerals formed as ancient seas evaporated Earth Science Chapter 3 27 Geode • Rounded hollow rock that is lined with mineral crystals • Formed when solutions of minerals seep into the rock through a crack and then the water evaporates Earth Science Chapter 3 28 Hot water solutions • Hot water dissolves minerals • The solution flows through cracks in other rocks • As the water cools, crystallization occurs • A vein is formed • Narrow channel or slab of a mineral that is different from the surrounding rock Earth Science Chapter 3 29 Discuss • What is crystallization? • What factor affects the size of crystals that form as magma cools? • What is a solution? • What are two ways in which minerals can form from a solution? Earth Science Chapter 3 30 Gemstones • Hard, colorful minerals with brilliant or glassy luster • Valuable minerals • Once polished, called gems • Used for decoration, mechanical parts, grinding and polishing Earth Science Chapter 3 31 Metals • Not as hard as gemstones • Can be made into wires, thin sheets, hammered, and molded without breaking • Conduct heat and electricity well Earth Science Chapter 3 32 Other uses • • • • • • Medicines Fertilizers Talc – baby powder Gypsum – sheetrock, cement, stucco Calcite – optical instruments Quartz – glass, watches, electronics Earth Science Chapter 3 33 Ore • Rock containing minerals to be mined and sold Earth Science Chapter 3 34 Producing metals from minerals • Prospecting – searching for ore • Can use magnetic field for iron, nickel, and cobalt • Mining – getting ore out of ground • Strip mines, pit mines, shaft mines • Smelting – getting metals out of ore • Use density differences to separate • Additional processing • Remove impurities • Make alloys • Solid mixtures of a metal and another element Earth Science Chapter 3 35 Discuss • What are gemstones? Why are they valuable? • What is an ore? • What properties of metal make them useful to humans? • If you were making a machine with lots of small, moving parts that must run constantly, would you make it out of metal or gemstones? Explain. Earth Science Chapter 3 36