Electrons in Atoms
advertisement

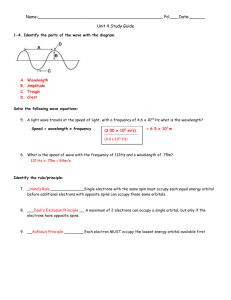
Electrons in Atoms Chapter 5 Electromagnetic Radiation light exhibits wave-like behavior as it travels through space Parts of the Wave Wavelength (λ) – shortest distance between equal points on a wave - crest to crest - units – m, cm, or nm Frequency (ν) – number of waves that pass by a given point every second - units – Hertz (Hz) or s-1 Electromagnetic Waves All electromagnetic waves travel at a speed of 3.00 x 108 m/s in a vacuum Speed of light is c = 3.00 x 108 m/s Speed is a product of wavelength and frequency c=λ·ν Wavelength and frequency are inversely related. As one increases the other decreases Wavelength vs. Frequency They are inversely related: Calculating Wavelength and Frequency You can find wavelength or frequency by using the speed of light equation c=λ·ν Ex. 1 – if the frequency of a microwave is 3.44 x 109 solve for the wavelength. 3.00 x 108 m/s = λ · 3.44 x 109 Hz 8 3.00 x10 m / s λ= = 0.0872m 9 3.44 x10 Hz Sample Problems c = λ · ν Ex. 2 - What is the wavelength of a ultraviolet ray that has a frequency of 4.80 x 1017 Hz? c=λ·ν Sample Problems c = λ · ν c=λ·ν 3.00 x 108 m/s = λ · 4.80 x 1017 Hz λ= 8 3.0 x10 m / s = 6.25 x 10-10m 17 4.80 x10 Hz Sample Problems c = λ · ν Ex. 3 - What is the frequency of a gamma ray that has a wavelength of 7.3 x 10-9m? c=λ·ν Sample Problems c = λ · ν c=λ·ν 3.00 x 108 m/s = 7.3 x 10-9 m · ν 8 3 . 00 x 10 m / s ν= = 4.10 x 1016 Hz 9 7.3x10 m Electromagnetic Spectrum Electromagnetic radiation includes radio waves that carry broadcasts to your radio and TV, microwave radiation used to heat food in a microwave oven, radiant heat used to toast bread, and the most familiar form, visible light. All of these forms of radiant energy are parts of a whole range of electromagnetic radiation called the electromagnetic spectrum Electromagnetic Spectrum Homework: ch. 5 # 1- 4 # 7, 12 pg. 121 pg. 126 Atomic Orbitals Electrons travel around the nucleus in definite orbitals (not on a fixed path) Electrons are on different energy levels – the higher the energy level the farther the electron is from the nucleus Atomic Orbitals Principle Energy Level Sublevel 1 s s, p s, p, d s, p, d, f 2 3 4-7 Energy Levels are divided into sublevels and orbitals s – orbitals are shaped like a sphere p – orbitals are shaped like dumbbells • Energy level 1 is closest to the nucleus and has the least amount of energy Atomic Orbitals Each orbital can hold two electrons s - 1 orbitals p – 3 orbitals d – 5 orbitals f – 7 orbitals (2 electrons) (6 electrons) (10 electrons) (14 electrons) Electron Configurations 1. 2. 3. The arrangement of electrons in an atom Three principles for filling in electron configurations Aufbau Principle Pauli Exclusion Principle Hunds Rule Electron Configuration Aufbau Principle - each electron occupies the lowest energy orbital available - energy diagram pg. 135 Electron Configurations Pauli Exclusion Principle - we use arrows to represent electrons - electrons spin in two different directions (up and down) ↑↓ - maximum of two electron per orbital Electron Configurations Hund’s Rule - single electrons w/ the same spin must occupy each orbital in a sublevel before additional electrons with opposite spins can occupy the same orbital - do example on board Orbital Diagrams Represent an atom’s electron configuration by drawing a box for each orbital and arrows for the electrons Electron Configuration Notation Designates the principle energy level 1, 2, 3….7 Designates the energy sublevel s, p, d, or f Includes a superscript representing the # of electrons in each sublevel Examples C – 1s2 2s2 2p2 N - 1s2 2s2 2p3 Electron Configuration Notation sample problems Na – 1s2 2s2 2p6 3s1 2+2+6+1 = 11 1s2 2s2 2p6 3s2 3p3 2+2+6+2+3 = 15 P– Br – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 Rb 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 Noble Gas Configuration Ne – 1s2 2s2 2p6 Na – 1s2 2s2 2p6 3s1 Al - 1s2 2s2 2p6 3s2 3p1 Use Noble gases for a shorter way to write out the electron configuration Use brackets to represent the noble gas and then add in the rest of what you need Na – [Ne] 3s1 Al – [Ne] 3s2 3p1 Noble Gas Configuration sample problems K– [Ar] 4s1 Pt – [Xe] 6s2 4f14 5d8 N– [He] 2s2 2p3 Valence Electrons The electrons in the outermost energy level are called valence electrons Valence electrons are the electrons that are involved in chemical bonding and determine the properties of an element All representative elements in the same group have the same number of valence electrons Valence Electrons Write the e- configuration for the following: P, Be, O, Al, Cl, and Ar P- 1s2 2s2 2p6 3s2 3p3 Be – 1s2 2s2 O – 1s2 2s2 2p4 Al – 1s2 2s2 2p6 3s2 3p1 Cl – 1s2 2s2 2p6 3s2 3p5 Ar – 1s2 2s2 2p6 3s2 3p6 - 5 v.e. - 2 v.e. - 6 v.e. - 3 v.e. - 7 v.e. - 8 v.e. Valence Electrons The elements number of valence electrons is equal to their group A # An element may never have more than eight valence electrons Because valence electrons are so important to the behavior of an atom, it is useful to represent them with symbols (Dot Diagrams) Dot Structures The symbol of the element with dots around it to represent the valence electrons (no more than 8 dots) Fill one dot on each side and then put two on all sides (no more than 8 dots) Dot Structures Write a Lewis dot diagram for each of the following. 1. Chlorine 7 v.e. 2. Calcium 2 v.e. 3. Potassium 1 v.e. Dot Structure of the Representative Elements Dot Structure of the Representative Elements