Ch.21Pt.2_000
advertisement

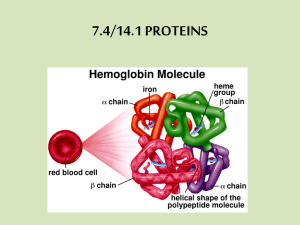
Tertiary Structure • A result of interactions between side (R) chains that are widely separated within the peptide chain Covalent disulfide bonds - between 2 cysteine AA Salt bridges - between AA w/ charged side chains (acid & base AA) Hydrogen bonds - between AA with polar R groups Hydrophobic attractions - between NP side chains • Spatial relationship of 2˚ structures • Level responsible for 3-D orientation of proteins. • Thermodynamically most stable conformation protein. • May have intra-chain and inter-chain linkages of a Tertiary protein structure bonding Human insulin, a small two-chain protein: Tertiary structure has both intra-chain & inter-chain disulfide linkages. 3o protein structure - Non-covalent R group interactions: (a) electrostatic interaction (b) hydrogen bonding (c) hydrophobic interaction Tertiary structure of the single-chain protein: myoglobin. found mainly in muscle tissue where it serves as an intracellular storage site for oxygen Quaternary structure: Shape or structure from joining more than one protein molecule (protein subunits) together to make a larger protein complex. Same non-covalent bonds as tertiary form: •Electrostatic interactions (Van der Waals) •Hydrophobic interactions •Hydrogen bonding Quaternary structure is easily disrupted Tertiary and quaternary structure of the oxygen-carrying protein hemoglobin. When O2 binds to Fe of Heme group, tension on the molecule pulls an amino acid, which alters the 3o structure – This in turn affects the 4o structure bonds Exposes more heme sites creates greater affinity for O2 Protein Structure R eview Review part 1: can you… •List the characteristics of proteins •Draw the basic structure of amino acids (a.a.) •Compare & contrast structural differences between the 4 main classes of a.a. •Draw a peptide formation between a.a. •List characteristics of four levels of protein structure (1o, 2o, 3o, and 4o) Types of Proteins • Two major types - based on structural levels – Fibrous - peptide chains are arranged in long strands/sheets – Globular - peptide chains are folded into spherical/globular shapes Fibrous versus Globular protein Fibrous Proteins Have fiber-like structures – good structural material. Relatively insoluble in water. Unaffected by moderate in temp and pH. Subgroups within this category include: Collagens & Elastins: the proteins of connective tissues. tendons and ligaments. Keratins: proteins that are major components of skin, hair, feathers and horn. Fibrin: a protein formed when blood clots. Myosin: a protein that makes up muscle tissue Globular Proteins In living organisms: Serve regulatory, maintenance and catalytic roles. Include hormones, antibodies, and enzymes. Either dissolve or form colloidal suspensions in water. Generally more sensitive to temperature & pH change than fibrous protein counterparts. Examples within this category include: Insulin Regulatory – controls glucose levels Hemoglobin Transport – moves O2 around body Myoglobin Storage – stores O2 near muscles Transferrin Transport – moves Fe in blood Immunoglobulins Defense – attacks invading pathogens Fibrous structural protein: Keratin Nails Horn & Hoof feathers Hair Keratin structural molecules are normally long and thin, insoluble in water, very high tensile strength,and arranged to form fibers. Composed of long rods, twisted together, laid down in criss-cross matrix form. Keratinized stratified squamous epithelial layer: found only in skin! Dead cell layers at surface. Keratin effectively waterproofs cells. Blocks diffusion of nutrients & wastes. Provides protection against friction, microbial invasion, and desiccation. Many cross-links create very little flexibility: horns, claws, hooves, or nails. Fewer cross-links allows some stretching but returns to normal: wool, skin, and muscle proteins. Fibrous structural protein: Collagen •Collagen most abundant protein in human body •Structural protein •Major component of the connective tissue: •sheaths muscles & attaches them to bone through tendons •or attaches skeletal elements together through cartilage Collagen exists as a molecule that is tightly coiled about itself forming a secondary triple coil. The molecules bunch together in groups of three, forming a larger coil (superhelical coil) that gives collagen fibers their strength in living tissue. Tendons Collagen structure can be disrupted in diseases such as scurvy, which is a lack of ascorbic acid, a cofactor in the hydroxylation of proline (Hydroxyproline) In addition, collagen structure is disrupted in rheumatoid arthritis. Myosin & Actin • Muscle proteins which allow for contraction of the muscle. • Myosin – Fibrous tail - two coiled -helices – Globular head - one at the end of each tail • Actin – A multimeric protein – Long fiber of connected globular proteins Muscle tissue contracts and relaxes when triggered by electrical stimuli from brain. Muscle fibers bundled together make up a single muscle. Many myofibrils make up each fiber. Myofibrils have striations, formed by arrangements of protein molecules. The protein forms filaments. 2 types of filament: thick & thin. Thick filaments contain myosin; thin filaments contain actin, troponin and tropomyosin.