A Brief History of AP® Chemistry (aka your Fall Semester study
advertisement

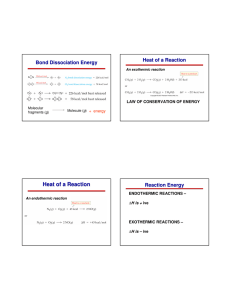
A Brief History of AP® Chemistry (aka your Fall Semester study guide) If the topic is preceded by a “1”, then you will need to memorize the facts for this topic If the topic is preceded by a “2”, then you will need to calculate a problem within this topic 1&2 – SI units of measurement 1 – Naming Compounds 1 – Polyatomic ion names and charges 1 – Classifications on the Periodic table (Halogens, Noble gases, et al) 2 – Stoichiometry a. Products and reactants created or used b. Limiting/excess reactant 1 – Types of Reactions 1&2 – Solutions a. 1 – Strong vs. weak electrolytes b. 2 – Concentrations of ions c. 2 – Dilutions d. 2 – Neutralizations 1&2 – Redox (Complete Step-by-step balancing of reactions) 2 – Gases – Gas Laws and Gas Stoichiometry 2 – Thermochemistry a. 2 – Internal energy b. 2 –Enthalpy c. 2 – Enthalpy changes (Hess’ Law) d. 2 – Q (heat gained or lost) e. 2 – Heat of formation 1&2 – Periodicity a. 2 – EM Radiation b. 1 & 2 – Frequency vs. wavelength c. 1 – Quantum #’s d. 1 – Electron configurations, Aufbau (yay!), and ALL periodic trends 1&2 – Bonding a. 1 – Polarity b. 2 – Type of Bond (ionic, polar, non-polar) c. 1 (sort of 2) – Lewis Dot Structures d. 2 – Bond Energies e. 2 – VSEPR – (bond angles and shape; be prepared to draw this) f. 1 – Resonance 1&2 – Hybridization of orbitals