Minerals
advertisement
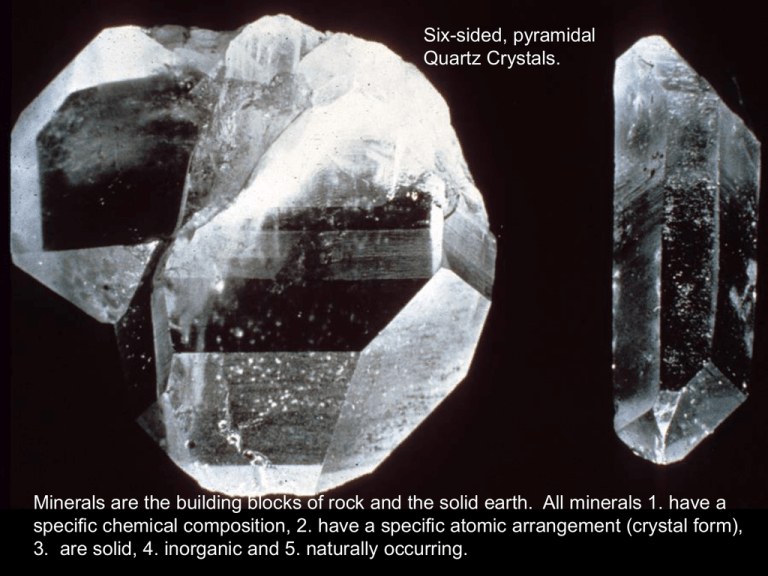
Six-sided, pyramidal Quartz Crystals. Minerals are the building blocks of rock and the solid earth. All minerals 1. have a specific chemical composition, 2. have a specific atomic arrangement (crystal form), 3. are solid, 4. inorganic and 5. naturally occurring. Basalt is composed of minerals that crystallize from magma derived from a partial melt of the asthenosphere. Crystals are too small to see with the naked eye. Minerals can form during crystallization of a magmatic melt. Andesite is composed of minerals that crystallize from magma derived from a partial melt of basaltic ocean crust. The large plagioclase crystals cooled slowly underground. Minerals can precipitate directly from an aqueous (out of water) solution, such as the salt deposits shown in the image above in the desert playa lake. The arrangement of the constituent atoms will define the crystal form of a mineral, providing the crystal can grow in an unrestricted environment. Quartz crystal Note that the quartz crystals in this granite do not form well-defined pyramidal shaped crystals because quartz is the last mineral to crystallize and inhibited by space. Chemical bond strength and the fixed arrangement of atoms will define whether weakness (cleavage planes) will form in minerals. The halite (salt) crystal shown in the image on the left has three cleavage planes at right angles (90°) to one another. The weakness planes develop between the ionic bonds between the sodium (Na) and chloride (Cl) atoms in the crystal lattice. Atoms are the smallest particles that define the chemical properties of matter. They are composed of protons (+ yellow) and neutrons (neutral orange) in the nucleus and electrons (-) surrounding the nucleus in defined energy levels. +1 +2 +3 +4 -2 -1 I have provided the common ionic charges for elements that are common in earth rocks. Note that N and P and metallic elements can have more than one valency state. +1 +2 +3 +4 -2 -1 I have provided the common ionic charges for elements that are common in earth rocks. Note that N and P and metallic elements can have more than one valency state. Covalent Bond Covalent Bond +1 +2 +3 +4 -2 -1 I have provided the common ionic charges for elements that are common in earth rocks. Note that N and P and metallic elements can have more than one valency state. The relative hardness of a mineral is controlled by its composition and bond strength between its constituent atoms. What mineral do you think is found on a dentist’s drill? Graphite and diamond have the same composition, but different atomic arrangements. The bonds between the carbon sheets in graphite are weak Van der waal bonds and it is very soft. Diamond is also composed of carbon atoms, but they are arranged in a more compacted structure than graphite and have strong covalent bonds between the carbon atoms. Diamond is the hardest natural mineral. Streak is most diagnostic for metallic minerals. Hematite (Fe2O3) leaves a distinct reddish-brown streak on a porcelain plate. Silicon and oxygen are the dominant elements comprising earth rocks. The other common elements are all cations (have positive ionic charges. The silicate tetrahedron is a complex anion with a charge of -4 (SiO4-4). It achieves charge balance and fulfills valencies by ionic and metallic bonding with available cations or covalent bonds between oxygen atoms of adjacent tetrahedra. The olivine structure satisfies its tetrahedral valencies with ionic and metallic bonds with magnesium (Mg+2) and iron (Fe+2) atoms. It is the first silicate mineral to crystallize from a magmatic melt. Olivine has no cleavage planes and will break along fractures. (Mg,Fe)2SiO4 Pyroxene is a single chain silicate that share two oxygen atoms. Pyroxene has two cleavage planes (at right angles). Can you infer which atomic bonds will define these cleavage planes? (Mg,Fe,Ca,Na)(Mg,Fe,Al)Si2O6 Pyroxene is one of the main minerals comprising basaltic ocean crust. The amphibole structure shares electrons between 2 or 3 of its oxygen atoms in its crystal structure. It will form two cleavage planes (124° and 56°) within the crystal structure. Note that water is also present in the crystal structure. (Na,Ca)2(Mg,Al,Fe)5(Si,Al)8O22(OH)2 Biotite: K2(Mg,Fe)6Si3O10(OH)2 Muscovite: K2Al4(Si6Al2O20)(OH,F)2 Muscovite and biotite micas are sheet silicates and have one cleavage plane at 180°. Based on the relative bond strength between constituent atoms within the crystal lattice can you predict where this cleavage plane forms? Biotite contains Fe and Mg which gives it a dark appearance. Both micas have dissolved water present. The 3-dimensional framework silicates share electrons between four of the oxygen atoms in each tetrahedron (covalent bonds exist between all tetrahedral oxygen atoms). Quartz has no cleavage planes and will break by conchoidal fracture. Note that the planes shown in the image above are crystal faces and do not repeat throughout the crystal. (Ca,Na)AlSi3O8 Plagioclase Feldspar The feldspar structure is similar to quartz except an aluminum (Al+3) will exchange with a silicon atom (Si+4) within the tetrahedron and require an additional electron to satisfy the extra valency. This bond can be between calcium (Ca+2), sodium (Na+1) and potassium (K+1), depending upon composition of the magma and crystallization temperature. KAlSi3O8 Potassium Feldspar Other important mineral groups include the carbonates of which calcite belongs. Calcite comprises limestone and marble. Halides (e.g., halite, NaCl) also form an important mineral group which sustains our lives. Galena (PbS) is a mineral in the sulfide group. It is an important source of the world’s lead. Hematite (Fe2O3) is a mineral in the oxide group. It is an important source of the world’s iron.






