Unit 8A Acid Base Homework
advertisement

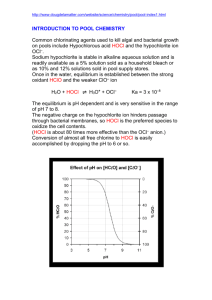
Unit 8A Acid Base Homework AP Directions: For each question, provide your work and final answer below the question. Without work, no credit will be given. Check significant figures and units. 1. H3PO2,H3PO3, and H3PO4 are monoprotic, diprotic and triprotic acids, respectively, and they are about equal strong acids. HClO2, HClO3, and HClO4 are all monoprotic acids, but HClO2 is a weaker acid than HClO3 which is weaker than HClO4. Account for: (a) The fact that the molecules of the three phosphorus acids can provide different numbers of protons. (b) The fact that the three chlorine acids differ in strengths. 2. A solution is prepared from 0.0250 mole of HCl, 0.10 mole propionic acid, C2H5COOH, and enough water to make 0.365 liter of solution. Determine the concentrations of H 3O+, C2H5COOH, C2H5COO–, and OH– in this solution. Ka for propionic acid = 1.3×10–5 NH4+ + OH– ↔NH3 + H2O H2O + C2H5O– ↔ C2H5OH + OH– The equations for two acid–base reactions are given above. Each of these reactions proceeds essentially to completion to the right when carried out in aqueous solution. (a) Give the Brønsted–Lowry definition of an acid and a base. (b) List each acid and its conjugate base for each of the reactions above. (c) Which is the stronger base, ammonia or the ethoxide ion. C2H5O–? Explain your answer. H2SO3 HSO3– HClO4 HClO3 H3BO3 3. Oxyacids, such as those above, contain an atom bonded to one or more oxygen atoms; one or more of these oxygen atoms may also be bonded to hydrogen. (a) Discuss the factors that are often used to predict correctly the strengths of the oxyacids listed above. (b) Arrange the examples above in the order of increasing acid strength. Unit 8A Acid Base Homework AP 4. Give a brief explanation for each of the following. (a) For the diprotic acid H2S, the first dissociation constant is larger than the second dissociation constant by about 105 (K1 ~ 105 K2). (b) In water, NaOH is a base but HOCl is an acid. (c) HCl and HI are equally strong acids in water but, in pure acetic acid, HI is a stronger acid than HCl. (d) When each is dissolved in water, HCl is a much stronger acid than HF. 5. The following observations are made about reaction of sulfuric acid, H2SO4. Discuss the chemical processes involved in each case. Use principles from acid–base theory, oxidation–reduction, and bonding and/or intermolecular forces to support your answers. (a) When zinc metal is added to a solution of dilute H2SO4, bubbles of gas are formed and the zinc disappears. (b) As concentrated H2SO4 is added to water, the temperature of the resulting mixture rises. (c) When a solution of Ba(OH)2 is added to a dilute H2SO4 solution, the electrical conductivity decreases and a white precipitate forms. Unit 8A Acid Base Homework AP Directions: Read the passage below and answer the summary questions that follow in complete sentences. Swimming Pool Chemistry Chem Matters ACS.org Those who own or maintain swimming pools know that frequent checks should be made on the water quality. One kind of pool "housekeeping" involves the removal of suspended particles such as leaves, dirt, and hair using skimmers and filters. A second kind deals with the much less visible buildup in the water of dissolved pollutants. Dissolved pollutants such as body wastes, algae, and disease-causing bacteria require chemical treatment. The chemical treatment of swimming pools involves the active disinfectant hypochlorous acid, HOCI. HOCI, a substance also used in drinking water purification and the final step of wastewater treatment, can be produced by the reaction of chlorine gas, C12, with water: Cl2(g) + H2O(1) HOCl (aq) + H+(aq) + Cl-(aq) Because of the corrosive and toxic properties of chlorine gas, sophisticated equipment is needed to' handle it. This makes it impractical for home swimming pool use. Therefore, chlorine-containing compounds that serve as a source of HOCI are used instead. Sodium hypochlorite, NaOCI, the active ingredient in household bleach, is a commonly used disinfectant because it reacts with water to produce HOCI: NaOCl (aq) + H2O HOCl (aq) + Na+(aq) + OH-(aq) Other examples of pool sanitizers are calcium hypochlorite, Ca(OCl)2 which is marketed as HTH® and chlorinated isocyanurates, such as trichloroisocyanuric acid. Ca(OCl)2 + 2 H2O 2 HOCl + Ca(OH)2 HOCI is a small molecule that is deadly to bacteria. Because of its size and lack of charge, it can easily penetrate the cell wall of a bacterium. Once it is inside, both the chlorine and the oxygen from the hypochlorous acid molecule oxidize or "burn out" the interior of the bacterium by breaking down the bacterium's protein. The amount of HOCI available in a swimming pool depends on several factors. Immediately after treatment, there is plenty of HOCI in a pool. The level of HOCI decreases as it is used in destroying bacteria, algae, and other organic substances in the pool. Also, the amount of HOCI present in the water depends on the pH of the water in the pool. To see how changes in pH affect the amount of HOCI available, we must understand that HOCI dissociates (breaks apart) to form hydrogen ion, H+, and hypochlorite ion, OCI-, in water: HOCl(aq) H+(aq) + OCl-(aq) (1) H+ and OCI- can also recombine to produce HOCI molecules: H+ (aq) + OCl- (aq) - HOCl (aq) (2) Because reaction 2 is just the reverse of reaction 1, and because both reactions are occurring at the same time, chemists usually write one equation to represent both processes. Unit 8A Acid Base Homework AP HOCl(aq) ↔ H+(aq) + OCI-(aq) When these two reactions occur at the same rate, we say that an equilibrium exists. When the pH of the water in the pool is lowered, that is when more H+ is added to the system, the extra H+ reacts with some of the OCl- already present to produce more HOCl. The concentration of HOCI available in the pool is increased and we say "the equilibrium is shifted to the left." If the pH is raised by adding a base, the extra hydroxide ion, OH-, combines with some of the H+ in the pool to produce water: H+(aq) + OH-(aq) H2O(l) Some of the available HOCl in the pool then breaks apart to form more H+ (to compensate for the H+ that was used up by the OH-) and more OCl-. We say that the "equilibrium is shifted to the right." Figure 1 shows how shifts in pH change the concentrations of OCl and HOCl. The ideal equilibrium distribution is equal concentrations of HOCl and OCl-. The table shows that a pH of 7.5 maintains this balance. If the pH is held in the range from 7.2 to 7.8, a suitable distribution of HOCl and OCl is provided. If the pH is lower than 7.2, the high concentration of HOCI is very irritating to the eyes of swimmers. Also, the growth of algae flourishes in this acid range. If the pH is higher than 7.8, too much of the disinfectant is present as OCI-, which is decomposed rapidly by sunlight The pH is adjusted by adding acid or base to the pool water. If the pH is too high (if the pool water is too basic), hydrochloric (muriatic) acid, HCl, or sodium bisulfate, NaHSO4 , can be added to the water to react with this excess base. If the pH is too low (if the pool is too acidic), sodium carbonate, Na2CO3, added to the pool will react with the excess acid and bring the pH back up to an acceptable value. Pool care involves both physical and chemical treatments. Although the tests used to determine the necessity of chemical treatment do not require an understanding of the chemistry involved, some knowledge of acid-base chemistry, pH, and equilibrium concepts provides the pool owner with the logic behind these chemical treatments. This knowledge also helps to ensure the safety of all who use the pool. Questions: 1. Why do we use a compound containing chlorine rather than pure chlorine in swimming pools? 2. After the initial addition of HOCl, explain why the concentration decreases over time. Unit 8A Acid Base Homework AP 3. The equilibrium created by the HOCl creates a solution known as a buffer. A buffer resists changes in pH when an acid or base is added. Why is this buffer solution essential in a pool? 4. If the pH is too low in a pool what are the hazards? 5. If the pH is too high in a pool what are the hazards? 6. Look up the Ka of HOCl and determine the pH of a 1.0M solution. 7. Will the pool have anywhere near a 1.0M HOCl concentration? I wish it was summer