IonicAndCovalentBonding
advertisement

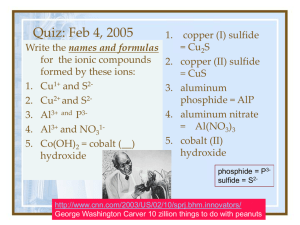
Bonding p. 97-106 • chemical bond: interaction between atoms or ions –decreases the potential energy of an atom; makes it more stable –only the valence electrons are involved! Three Types of Bonds • Bond type depends on electronegativity (electron affinity) –IONIC:very different electronegativities • one metal, one non-metal –COVALENT: both very high • two non-metals –METALLIC: both low • one or more metals Review of Ionisation +1 +2 -3 -2 -1 Anions Cations Metals Nonmetals What kind of bond? • • • • • • • • Na and Cl O and F K and Br Au and Ag H and O Mg and F S and Cl Ni and Cu • • • • • • • • Ionic Covalent Ionic Metallic Covalent Ionic Covalent Metallic Ionic Bonds • metal (low electroneg., cation) + nonmetal (high electroneg., anion) • Array of positively and negatively charged ions –held together by electrostatic attraction • metal loses e- = cation –group 1 (+1) and group 2 (+2) –transition metals (+2 most common) • non-metal gains e- = anion –groups 17, 16, 15… • List, p. 100 sodium and chlorine Na Cl • sodium transfers its electron to chlorine Crystal Lattice (Array) • structure of an ionic bond • each anion is surrounded by cations and vice versa Writing Formulae for Ionic Compounds • chemical formula: shorthand for elements, ions and compounds • Ratio of the number of atoms of each element –MgCl2 –C6H12O6 • Ions of opposite charges are attracted to one another. • Mg2+ Cl• MgCl2 • Ions bond because they are electrically attracted to one another –“Opposites attract” • Polyatomic ions: most covalently bonded, but have an overall electronic charge • Hand out list: memorize it. Ionic or Covalent? • • • • • NaCl NO2 N2Br NaI CaS Ionic or Covalent? • • • • • KNO3 Fe(CrO4)2 Cu(OH)2 BaI F2 Ionic or Covalent? • O2 • AgCl • AgNO3 Ionic or Covalent? • • • • • NO2 CO2 PCl5 P2S4 NO3 Writing Formulae for Ionic Compounds • Write the symbols and their charges • “Cross” the charges to the other side • Use the charges, without + or – as subscripts Polyatomic Ions to Memorize • • • • • • • • Ammonium Nitrite Nitrate Sulfite Sulfate Hydroxide Phosphite phosphate • • • • Carbonate Chlorite Chlorate Chromate Naming Ionic Compounds Naming Cations • Same as the element!! Naming Anions • • • • • • • Ending changes to “ide” O oxygen oxide F fluorine fluoride S sulfur sulfide Cl chlorine chloride Br bromine bromide I iodine iodide • • • • • • Chlorine Iodine Oxygen Sulfur Bromine Fluorine • • • • • • Chloride Iodide Oxide Sulfide Bromide Fluoride Naming Ionic Compounds • Cation + Anion (“ide” ending) • NaCl sodium chloride • KBr potassium bromide Naming Ionic Compounds – Type I • One positively charged ion and one negatively charged ion. • NaCl –Sodium chloride • SrF2 –Strontium fluoride 1. 2. 3. 4. 5. 6. 7. 1. CsBr 2. MgO 3. KF 4. AlCl3 5. LiH calcium iodide 6. rubidium sulfide 7. cesium bromide magnesium oxide potassium fluoride aluminum chloride lithium hydride CaI2 Rb2S • • • • • • • LiI CaS AgBr ZnCl2 Na2S barium fluoride silver oxide • • • • • • • lithium iodide calcium sulfide silver bromide zinc chloride sodium sulfide BaF2 Ag2O Stock system • Some elements make ions with different charges (p. 100) –“oxidation states” • Fe2+ Fe3+ • iron(II) iron(III) • Roman numerals Elements that use the Stock System • These elements have more than one “oxidation state” –Fe (2+, 3+) Cr (2+, 3+) –Cu (1+, 2+) Mn (2+, 3+) –Co (2+, 3+) Pb (2+, 4+) –Sn (2+, 4+) –Hg2+ (mercury II), Hg22+ (mercury I) –Zumdahl, p. 65 Ions to memorize • • • • Al3+ Zn2+ Ag+ Cd2+ 1. 2. 3. 4. 5. 6. 7. • copper (I) chloride CuCl • tin (II) oxide SnO • iron (III) oxide Fe2O3 • manganese (IV) oxide MnO2 • lead (II) chloride PbCl2 • Cu2O3 copper (III) oxide vanadium (IV) fluoride • VF4 1. 2. 3. 4. 5. 6. CoBr2 CrCl3 CaCl2 Al2O3 SnBr4 Cu2S cobalt (II) bromide chromium (III) chloride calcium chloride aluminum oxide tin (IV) bromide copper (I) sulfide 7. iron (II) fluoride • FeF 2 8. tin (II) oxide • SnO • • • • • • • Fe(NO3)3 –Iron(III) nitrate • Fe2(SO4)3 –iron(III) sulfate Polyatomic Ion Ionic Compounds with Polyatomic Ions • NH4+ –ammonium • NO3–nitrate 1. 2. 3. 4. 5. 6. 7. Na2SO4 1. KH2PO4 2. Fe(NO3)3 3. Mn(OH)2 4. Na2SO3 5. Rb2CO3 6. Mg(HCO3)27. sodium sulfate potassium dihydrogen phosphate iron (III) nitrate manganese (II) hydroxide sodium sulfite rubidium carbonate magnesium bicarbonate • • • • • • Name Ionic Compounds potassium nitrate magnesium hydroxide lithium chromate iron (III) hydroxide cobalt nitrate Manganese (II) posphite • • • • • • KNO3 Mg(OH)2 LiCrO4 Fe(OH)3 Co(NO3)2 Mn3(PO3)2 Homework 1. CaO 2. lithium sulfide 3. CrCO3 4. silver iodide 5. Cu2SO3 6. calcium phosphate 7. SrI2 8. iron (III) bromide 9. FeBr2 10.cobalt (II) iodide 11.SnO2 12.ammonium nitrate 13.Ag2S Covalent Bonding Covalent Bonding • two or more non-metals • atoms share some valence electrons (not transfer) • single covalent bond: shares one pair of electrons • double: 2 pairs of e• triple: 3 pairs of e- • usually each atom donates (shares) one of each pair of electrons • dative covalent bond: sometimes one atom donates both electrons F F Electron Pair Shared by both atoms Each e- donate by each atom • number of bonds formed depends on the number of e- required to fill the valence shell –noble gases = full valence, rarely form compounds • octet rule: usually, atoms want 8 valence e- (H, He need 2) • Ex: C has 4 valence e–needs 4 more to form a full octet –C forms 4 bonds • Ex: F has 7 valence e–needs 1 more to form a full octet –F makes one bond • Nitrogen? Bonding between C and F F F F C F F C F F F structural formula hybrid diagram =e- pair =covalent bond F F C F F Lewis diagram F e - C e- Double Bond • sharing two pairs of electrons • bonds more strongly than a single bond O C O O C O O C O • structural hybrid Lewis Triple Bond • Strongest N N N N N N Length and Strength of Bonds single longest lowest energy double triple shortest highest energy Drawing molecule diagrams 1. Decide how many bonds each atom makes. 2. The central atom is the one that makes the most bonds. 3. Draw with single bonds 4. Calculate remaining electrons 5. Use remaining electrons • HCN (hydrogen cyanide) –C=4, N=3, H=1 • HCO2- (methanoate ion) –H=1, C=4, O=2 Draw structural, hybrid and Lewis structures • • • • • HF NH3 CH4 CF4 NO2- • • • • CHCl3 NH4+ H2CO SeF2 Naming Covalent Compounds • Ex: CO2 –carbon dioxide • Prefixes –mono 1 –di –tri –tetra –penta 5 –hexa 2 3 4 6 1. 2. 3. 4. 5. 6. N2O NO NO2 N2O3 N2O4 N2O5 1. 2. 3. 4. 5. 6. Phosphorus pentachloride Phosphorus trichloride Sulfur hexafluoride Sulfur trioxide Sulfur dioxide Carbon dioxide • • • • • P4O10 Fe2O3 Li2O2 Mg(NO3)2 CCl4 • • • • • tetraphosphorus decoxide iron (III) oxide lithium peroxide Magnesium nitrate Carbon tetrachloride Homework • • • • • NI3 SF2 N2O4 ICl3 SF6 phosphorus trichloride dinitrogen tetrafluoride sulfur dioxide diphosphorus pentasulfide dihydrogen monoxide