Lecture - Ch 8
advertisement

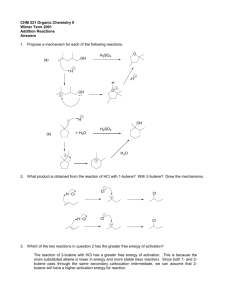
Chapter 8 Alkenes: Reactions and Synthesis Suggested Problems 1-21,26-9,32-5,37-39 CHE2201, Chapter 8 Learn, 1 Diverse Reactions of Alkenes • Alkenes undergo electrophilic addition reactions to give many useful products CHE2201, Chapter 8 Learn, 2 Preparation of Alkenes: A Preview of Elimination Reactions • Alkenes are commonly made by an elimination reaction 1.Dehydrohalogenation - Loss of HX from an alkyl halide • Occurs by reaction of an alkyl halide with strong base CHE2201, Chapter 8 Learn, 3 Preparation of Alkenes: A Preview of Elimination Reactions 2. Dehydration - Loss of water from an alcohol • Carried out by treating an alcohol with a strong acid CHE2201, Chapter 8 Learn, 4 Worked Example • How many alkene products, including E,Z isomers, might be obtained by dehydration of 3methyl-3-hexanol with aqueous sulfuric acid? CHE2201, Chapter 8 Learn, 5 Worked Example • Solution: – It is possible to obtain five alkene products by the dehydration of 3-methyl-3-hexanol CHE2201, Chapter 8 Learn, 6 Electrophilic Addition Reactions CHE2201, Chapter 8 Learn, 7 Halogenation of Alkenes: Addition of X2 • Halogenation - Bromine and chlorine add to alkenes to give 1,2-dihalides • Example – 1,2-dichloroethane is formed by addition of Cl2 to ethylene • Fluorine is too reactive and iodine does not react with majority of alkenes CHE2201, Chapter 8 Learn, 8 Halogenation of Alkenes: Addition of X2 • Halogenation reaction of cycloalkane forms the trans stereoisomer of the dihalide addition product • Reaction occurs with anti stereochemistry CHE2201, Chapter 8 Learn, 9 Mechanism of Bromine Addition • As suggested by George Kimball and Irving Roberts, for the observed stereochemistry the reaction intermediate is not a carbocation • Bromonium ion, R2Br+, is formed by electrophilic addition of Br+ to the alkene CHE2201, Chapter 8 Learn, 10 Mechanism of Bromine Addition • Bromonium ion is formed in a single step – Interaction of the alkene with Br2 and simultaneous loss of Br- • Reaction with Br- ion occurs only from the opposite, unshielded side to give trans product CHE2201, Chapter 8 Learn, 11 Mechanism of Bromine Addition • Bromonium ions were postulated more than 75 years ago to explain stereochemistry of halogen addition to alkenes • George Olah showed that bromonium ions are stable in liquid SO2 CHE2201, Chapter 8 Learn, 12 Addition of a Halogen is an Anti Addition CHE2201, Chapter 8 Learn, 13 Anti Addition to a Cis Isomer Forms Only the Trans Stereoisomers CHE2201, Chapter 8 Learn, 14 No Carbocation Rearrangements CHE2201, Chapter 8 Learn, 15 Worked Example • Addition of HCl to 1,2-dimethylcyclohexene yields a mixture of two products – Show the stereochemistry of each, and explain why a mixture is formed • Solution: • Addition of hydrogen halides involves formation of an open carbocation • The carbocation, which is sp2-hybridized and planar, can be attacked by chloride from either top or bottom CHE2201, Chapter 8 Learn, 16 Worked Example – This yields products in which the two methyl groups can be either cis or trans to each other CHE2201, Chapter 8 Learn, 17 Halohydrins from Alkenes: Addition of HOX • Reaction of alkenes with hypohalous acids HO–Cl or HO–Br yields 1,2-halo alcohol, called a halohydrin • Addition takes place by reaction of the alkene with either Br2 or Cl2 in the presence of water CHE2201, Chapter 8 Learn, 18 Formation of Halohydrins CHE2201, Chapter 8 Learn, 19 Mechanism for Halohydrin Formation CHE2201, Chapter 8 Learn, 20 How to Account for Regioselectivity The electrophile adds to the sp2 carbon bonded to the most hydrogens. CHE2201, Chapter 8 Learn, 21 Halohydrins from Alkenes: Addition of HOX • Bromohydrin formation is carried out in a solvent such as aqueous dimethyl sulfoxide, CH3SOCH3 (DMSO), using the reagent N-bromosuccinimide (NBS) – Produces bromine in organic solvents and is a safer source CHE2201, Chapter 8 Learn, 22 NBS Mechanism CHE2201, Chapter 8 Learn, 23 More Reactions • Different nucleophiles • Each involves a chloronium or bromonium ion intermediate CHE2201, Chapter 8 Learn, 24 Worked Example • What product would you expect from the reaction of cyclopentene with NBS and water? – Show the stereochemistry • Solution: – –Br and –OH are trans in the product CHE2201, Chapter 8 Learn, 25 Hydration of Alkenes: Addition of H2O • Hydration of an alkene is the addition of H2O to give an alcohol • Reaction takes place on treatment of the alkene with water and a strong acid catalyst CHE2201, Chapter 8 Learn, 26 Mechanism of Hydration CHE2201, Chapter 8 Learn, 27 Hydration of Alkenes: Addition of H2O • Acid-catalyzed hydration of isolated double bonds is uncommon in biological pathways • Fumarate is hydrated to give malate as one step in the citric acid cycle of food metabolism • In the laboratory, alkenes are often hydrated by the oxymercuration–demercuration procedure CHE2201, Chapter 8 Learn, 28 Hydration of Alkenes: Addition of H2O by Oxymercuration • Reaction is initiated by electrophilic addition of Hg2+ ion to the alkene – Gives an intermediate mercurinium ion • Regiochemistry of the reaction corresponds to Markovnikov addition of H2O CHE2201, Chapter 8 Learn, 29 Acid-Catalyzed Addition of an Alcohol CHE2201, Chapter 8 Learn, 30 Carbocation Rearrangement (a 1,2-hydride shift) CHE2201, Chapter 8 Learn, 31 Carbocation Rearrangement (a 1,2-methyl shift) CHE2201, Chapter 8 Learn, 32 The Carbocation Does Not Rearrange • No Improvement in Carbocation Stability is afforded here CHE2201, Chapter 8 Learn, 33 Worked Examples • What products would you expect from oxymercuration–demercuration of the following alkenes? a) b) CHE2201, Chapter 8 Learn, 34 Worked Examples • Solution: – Oxymercuration is equivalent to Markovnikov addition of H2O to an alkene a) b) CHE2201, Chapter 8 Learn, 35 Hydration of Alkenes: Addition of H2O by Hydroboration • Hydroboration: Process involving addition of a B–H bond of borane, BH3, to an alkene to yield an organoborane intermediate, RBH2 • Boron has six atoms in its valance shell making borane a very reactive Lewis acid CHE2201, Chapter 8 Learn, 36 BH3 Contains Three Hydrides – Dialkyl and Trialkylboranes Typically Form CHE2201, Chapter 8 Learn, 37 Hydration of Alkenes: Addition of H2O by Hydroboration • Alkene reacts with BH3 in THF solution, rapid addition to the double bond occurs three times and a trialkylborane is formed • Net effect of the two-step hydroboration– oxidation sequence is hydration of the alkene CHE2201, Chapter 8 double bond Learn, 38 R2BH Allows Only Monoalkylation Because of its bulky R groups, it has a stronger preference for the less substituted sp2 carbon. CHE2201, Chapter 8 Learn, 39 Hydration of Alkenes: Addition of H2O by Hydroboration • During hydroboration–oxidation of 1-methylcyclopentene, boron and hydrogen add to the alkene from the same face of the double bond with syn stereochemistry CHE2201, Chapter 8 Learn, 40 Hydroboration • Differs from other alkene addition reactions – Occurs in a single step without a carbocation intermediate CHE2201, Chapter 8 Learn, 41 Addition of BH3 and Addition of HBr Follow the Same Rule • Regiochemistry that results when an unsymmetrical alkene is hydroborated makes the hydroboration reaction very useful • Anti-Markovnikov CHE2201, Chapter 8 Learn, 42 Mechanism for the Oxidation Reaction CHE2201, Chapter 8 Learn, 43 Only Syn Addition CHE2201, Chapter 8 Learn, 44 The H and OH Add to the Same Side of the Ring • Hydroboration-oxidation is stereoselective – only two of the four possible stereoisomers are formed. CHE2201, Chapter 8 Learn, 45 No Carbocation Rearrangements CHE2201, Chapter 8 Learn, 46 Worked Example • What alkene might be used to prepare the following alcohol by hydroboration–oxidation? • Solution: – The products result from hydroboration/oxidation of a double bond – The –OH group is bonded to the less substituted carbon of the double bond in the starting material CHE2201, Chapter 8 Learn, 47 Retrosynthesis How would you make this molecule? CHE2201, Chapter 8 Learn, 48 Reduction of Alkenes: Hydrogenation • Hydrogenation: Addition of hydrogen to a double or triple bond to yield a saturated product • Reduction: Reaction that results in gain of electron density for carbon caused either by: – Bond formation between carbon and a less electronegative atom (usually H) – Bond-breaking between carbon and a more electronegative atom (usually O,N,X) CHE2201, Chapter 8 Learn, 49 Reduction of Alkenes: Hydrogenation • Usually occurs with syn stereochemistry • H2 is adsorbed onto a catalyst surface CHE2201, Chapter 8 Learn, 50 Mechanism for Hydrogen Addition catalytic hydrogenation CHE2201, Chapter 8 Learn, 51 Reduction of Alkenes: Hydrogenation • Catalytic hydrogenation is extremely sensitive to the steric environment around the double bond • In α-pinene reduction occurs exclusively from the bottom face CHE2201, Chapter 8 Learn, 52 Selectivity in Hydrogenation CHE2201, Chapter 8 Learn, 53 Reduction of Alkenes: Hydrogenation • Catalytic hydrogenation is important in the food industry • Incomplete hydrogenation results in partial cis– trans isomerization of a remaining double bond • In biological hydrations, biological reductions occur in two steps: – Reducing agent, NADPH, adds a hydride ion to the double bond to give an anion – Anion is protonated by acid HA, leading to overall addition of H2 CHE2201, Chapter 8 Learn, 54 Trans Fatty Acids Raise LDL CHE2201, Chapter 8 Learn, 55 Reduction of the Carbon–Carbon Double Bond in Trans-crotonyl ACP CHE2201, Chapter 8 Learn, 56 Worked Example • What products are obtained from catalytic hydrogenation of the following alkenes? a) b) CHE2201, Chapter 8 Learn, 57 Worked Example • Solution: a) b) CHE2201, Chapter 8 Learn, 58 Oxidation of Alkenes: Epoxidation and Hydroxylation • Oxidation: Reaction that results in a loss of electron density for carbon by: – Bond formation between carbon and a more electronegative atom (usually O,N,X) – Bond-breaking between carbon and a less electronegative atom (usually H) CHE2201, Chapter 8 Learn, 59 Oxidation of Alkenes: Epoxidation • Alkenes oxidize to give epoxides on treatment with a peroxyacid, RCO3H • Epoxide: Cyclic ether with an oxygen atom in a three-membered ring CHE2201, Chapter 8 Learn, 60 Only Syn Addition is Observed CHE2201, Chapter 8 Learn, 61 Mechanism for Epoxidation The mechanism is similar to that for the addition of Br2 CHE2201, Chapter 8 Learn, 62 Syn Addition to a Cis Isomer Forms Only the Cis Stereoisomers CHE2201, Chapter 8 Learn, 63 Syn Addition to a Trans Isomer Forms Only the Trans Stereoisomers CHE2201, Chapter 8 Learn, 64 Oxidation of Alkenes: Epoxidation • Treating a base with halohydrin leads to elimination of HX and production of an epoxide CHE2201, Chapter 8 Learn, 65 Oxidation of Alkenes: Hydroxylation • Epoxides undergo an acid-catalyzed ringopening reaction with water – Gives corresponding 1,2-dialcohol, or diol, also called a glycol CHE2201, Chapter 8 Learn, 66 Oxidation of Alkenes: Hydroxylation • The net result of the two-step alkene epoxidation/hydrolysis is hydroxylation CHE2201, Chapter 8 Learn, 67 Oxidation of Alkenes: Hydroxylation • Hydroxylation can be carried out directly by treating an alkene with osmium tetroxide – Reaction occurs with syn stereochemistry – Does not involve a carbocation intermediate CHE2201, Chapter 8 Learn, 68 Oxidation of Alkenes: Hydroxylation • The use of NMO as a cooxidant permits a catalytic cycle CHE2201, Chapter 8 Learn, 69 Worked Example • What product is expected from reaction of cis-2butene with meta-chloroperoxybenzoic acid (mCPBA)? – Show the stereochemistry CHE2201, Chapter 8 Learn, 70 Worked Example • Solution: – Epoxidation using m-chloroperoxybenzoic acid (mCPBA) is a syn addition – Original double bond stereochemistry is retained – The methyl groups are cis CHE2201, Chapter 8 Learn, 71 Oxidation of Alkenes: Cleavage to Carbonyl Compounds • Ozone (O3) adds to C═C bond, at low temperature, to form molozonide • Molozonide rearranges to form ozonide CHE2201, Chapter 8 Learn, 72 Oxidation of Alkenes: Cleavage to Carbonyl Compounds • Ozonide is treated with a reducing agent to produce carbonyl compounds (Zn/AcOH or dimethyl sulfide) CHE2201, Chapter 8 Learn, 73 Ozonolysis CHE2201, Chapter 8 Learn, 74 What Alkene Gave these Ozonolysis Products? CHE2201, Chapter 8 Learn, 75 Oxidation of Alkenes: Cleavage to Carbonyl Compounds • Oxidizing reagents other than ozone cause double-bond cleavage • Potassium permanganate (KMnO4) can produce carboxylic acids and carbon dioxide if hydrogens are present on C═C • With no hydrogens, ketones are produced CHE2201, Chapter 8 Learn, 76 Oxidation of Alkenes: Cleavage to Carbonyl Compounds • Alkenes can be cleaved by hydroxylation to form a 1,2-diol followed by reaction of the diol with periodic acid, HIO4, to afford carbonyl compounds CHE2201, Chapter 8 Learn, 77 Worked Example • What products would be expected from reaction of 1-methylcyclohexene with aqueous acidic KMnO4? • Solution: – Aqueous KMnO4 produces: • A carboxylic acid from a C═C • A ketone from a double bond carbon that is disubstituted CHE2201, Chapter 8 Learn, 78 Addition of Carbenes to Alkenes: Cyclopropane Synthesis • Carbene, R2C: A neutral molecule containing a divalent carbon with only six electrons in its valence shell – Electrophilic addition of a carbene to an alkene yields a cyclopropane • Adds symmetrically across the double bond CHE2201, Chapter 8 Learn, 79 Dichlorocarbene Generation Mechanism CHE2201, Chapter 8 Learn, 80 The Structure of Dichlorocarbene • A carbene is planar being sp2 hybridized with a vacant p-orbital. • Note its similarity in structure to that of a carbocation CHE2201, Chapter 8 Learn, 81 Addition of Carbenes to Alkenes: Cyclopropane Synthesis • Addition of dichlorocarbene with cis-2-pentene is stereospecific – Stereospecific: Only a single stereoisomer is formed as product CHE2201, Chapter 8 Learn, 82 Simmons-Smith Reaction • Method for preparing nonhalogenated cyclopropanes • Does not involve a free carbene • Utilizes a carbenoid • Reaction of diiodomethane with zinc-copper alloy produces (iodomethyl)zinc iodide CHE2201, Chapter 8 Learn, 83 Simmons-Smith Reaction • (Iodomethyl)zinc iodide yields the corresponding cyclopropane in the presence of an alkene CHE2201, Chapter 8 Learn, 84 Worked Example • What product is expected from the following reaction CHE2201, Chapter 8 Learn, 85 Worked Example • Solution: – Reaction of a double bond with CH2I2 yields a product with a cyclopropane ring that has a – CH2– group – Two different isomers can be formed, depending on stereochemistry of the double bond CHE2201, Chapter 8 Learn, 86 Radical Additions to Alkenes: Chain-Growth Polymers • Polymer: Large molecule consisting of repeating units of simpler molecules, called monomers – Formed by polymerization • Alkenes react with radical catalysts to undergo radical polymerization • Simple alkene polymers are called chaingrowth polymers CHE2201, Chapter 8 Learn, 87 Radical Additions to Alkenes: Chain-Growth Polymers CHE2201, Chapter 8 Learn, 88 Radical Additions to Alkenes: Chain-Growth Polymers • Initiation – A few radicals are generated on heating a small amount of benzoyl peroxide catalyst – Benzoyloxy radical loses CO2 and gives a phenyl radical CHE2201, Chapter 8 Learn, 89 Radical Additions to Alkenes: Chain-Growth Polymers • Propagation – Radical from initiation adds to alkene to generate alkene derived radical – Process repeats to form the polymer chain • Termination – Chain propagation ends when two radical chains combine CHE2201, Chapter 8 Learn, 90 Radical Additions to Alkenes: Chain-Growth Polymers • Other alkenes give other common polymers CHE2201, Chapter 8 Learn, 91 Some Alkene Polymers and Their Uses CHE2201, Chapter 8 Learn, 92 Worked Example • Show the monomer units required to prepare the following polymer: • Solution: – The smallest repeating unit in each polymer is identified and double bond is added – Monomer • H2C═CHOCH3 CHE2201, Chapter 8 Learn, 93 Biological Additions of Radicals to Alkenes • More controlled and more common than laboratory or industrial radical reactions • Radical addition reactions have severe limitations in a laboratory environment CHE2201, Chapter 8 Learn, 94 Pathway of Biosynthesis of Prostaglandins from Arachidonic Acid CHE2201, Chapter 8 Learn, 95 Reaction Stereochemistry: Addition of H2O to an Achiral Alkene • The laboratory hydration of 1-butene yields an intermediate secondary carbocation by protonation • It reacts with H2O from either the top or the bottom face to afford the two enantiomers • Formation of a new chirality center by achiral reactants leads to a racemic mixture of enantiomeric products CHE2201, Chapter 8 Learn, 96 Reaction of H2O with the Carbocation Resulting from Protonation of 1-Butene • The two transition states are mirror images CHE2201, Chapter 8 Learn, 97 Reaction Stereochemistry: Addition of H2O to an Achiral Alkene • Optically active product can only result by starting with an optically active reactant or a chiral environment • Cis-aconitate is achiral – Only the enantiomer of the product is formed in a biological reaction CHE2201, Chapter 8 Learn, 98 Reaction Stereochemistry: Addition of H2O to a Chiral Alkene • The stereochemistry in acid-catalyzed addition of H2O is established by reaction of H2O with a carbocation intermediate – Does not contain a plane of symmetry – Chiral because of existing chirality center • Formation of a new chirality center by a chiral reactant leads to unequal amounts of diastereomeric products • Products are also optically active, if the chiral reactant is optically active because only one enantiomer is used CHE2201, Chapter 8 Learn, 99 Stereochemistry of the Acid-Catalyzed Addition of H2O to the Chiral Alkene CHE2201, Chapter 8 Learn, 100 Worked Example 1 Which reaction would one predict to be faster, addition of HBr to cyclohexene or to 1methylcyclohexene? CHE2201, Chapter 8 Learn, 101 Worked Example 1 First, draw out both reactants with HBr. What we should realize at this point is that the formation of the intermediate that is more stabilized via carbocation formation is the one that will form product faster. At this point, we should see that the intermediate formed via the 3˚ intermediate from 1methylcyclohexene (as opposed to the 2˚ carbocation intermediate in the case of cyclohexene) will proceed faster. CHE2201, Chapter 8 Learn, 102 Worked Example 2 • What products are formed from hydration of 4methylcyclopentene? – Consider all stereoisomers formed CHE2201, Chapter 8 Learn, 103 Worked Example 2 • Solution: CHE2201, Chapter 8 Learn, 104