File
advertisement

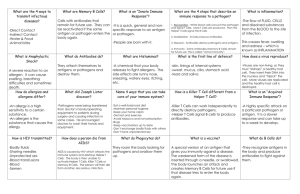
Chapter 43 The Immune System Recognition & Response Pathogens, agents (virus, bacteria, fungi) that cause disease, infect lots of animals. For them, the internal environment of the host animal is an ideal habitat. o Animal body offers ready source of nutrients, protected setting for growth & reproduction & means of transport to new environments Immune system- enables animal to avoid or limit many infections 2. Innate immunity- defense that’s active immediately upon infection & is the same whether or not the pathogen has been encountered previously o Ex: outer covering that provides a significant barrier to entry by microbes But sealing off entire body surface is impossible because gas exchange, nutrition & reproduction require openings to the environment o Ex: chemical secretion that trap or kill microbes guard the body’s entrances & exits o Ex: Linings of the digestive tract, airway, & other exchange surfaces If a pathogen breaks through barrier defenses and enters the body, an animal’s immune system must detect foreign particles & cells within the body to fight infections; a properly functioning immune system distinguishes nonself from self o Molecular recognition- receptor molecules bind specifically to molecules from foreign cells or viruses; accomplishes detection of nonself A small preset group of receptor proteins bind to molecules that are absent from animal bodies but present in antibodies. Binding of an innate immune receptor to a foreign molecule activates internal defenses 1. Adaptive (acquired) immunity- a type of molecular recognition only found in vertebrates; produce lots of receptors, each of which recognize a feature typically found only on a particular part of a particular molecule in a particular pathogen, so recognition & response occur with specificity o Ex: synthesis of proteins that inactivate a bacterial toxin o Ex: targeted killing of a virus-infected cell body Activated after the innate immune response & develops more slowly It is enhanced by previous exposure to the infecting pathogen 43.1 In Innate Immunity, Recognition & Response Rely on Traits Common to Groups of Pathogens Innate Immunity of Invertebrates Insects in terrestrial & freshwater habitats filled with diverse microbes shows the effectiveness of invertebrate innate immunity, as insects rely on their exoskeleton as a fist line of defense against infection o The exoskeleton is composed of the polysaccharide chitin & provides an effective barrier defense against most pathogens. a. Chitin-based barrier is also in insect’s intestine, where it blocks infection by many pathogens ingested with food i. Lysozyme- enzyme that breaks down bacterial cell walls; also protects insect digestive system If a pathogen breaches an insect’s barrier defenses, it encounters many internal immune defenses o Hemocytes are immune o Hemocytes, immune cells, travel throughout the body in the hemolymph (insect circulatory fluid) a. Some hemocytes carry out phagocytosis, a defense in which the cellular ingestion & digestion of foreign substance (i.e. bacteria) b. Other hemocytes trigger the production of chemicals that kill pathogens and help entrap large parasites (i.e. Plasmodium, the parasite of mosquitoes that causes malaria) c. Encounters with pathogens in hemolymph causes hemocytes & certain other cells to secrete antimicrobial peptides, short chains of amino acids that circulate throughout the body of the insect & inactivate or kill fungi & bacteria by disrupting their plasma membrane Immune cells of insects, which are not found in animal cells, bind to molecules found only in outer layers of fungi or bacteria o Fungal cell walls contain certain unique polysaccharides & bacterial cell walls have polymers containing combos of sugars & amino acids These macromolecules serve as identity tags for pathogen recognition Insect immune cells secrete specialized recognition proteins, each of which binds to a macromolecule characteristic of fungi or bacteria Innate immune responses are distinct for different classes of pathogens a. Ex: when fungus Nerosporar craesa infects a fruit fly, pieces of the fungal cell wall bind to a recognition protein. This complex activates the protein Toll, a receptor on the surface of hemocytes i. Signal transduction from the Toll receptor to the cell nucleus leads to synthesis of a set of antimicrobial peptides active against fungi b. Ex: if fruit fly is infected by bacterium Microoccus luteus, a different recognition protein’s activated, & the fly produces a different set of antimicrobial peptides c. It’s hard to pinpoint and target the activity of a specific peptide because fruit flies secrete many distinct antimicrobial peptides in response to a single infection i. Modern genetic techniques to reprogram the fly immune system helped discover that the synthesis of a single type of antimicrobial peptide in the fly’s body could provide an effective immune defense i. Brybi Kenautre began with a mutant fly strain in which pathogens are recognized but the signaling that would normally trigger innate immune response is blocked. So, the mutant flies don’t make any antimicrobial peptides. ii. Then, they genetically modified some of the mutant flies to express lots of a single antimicrobial peptide. iii. They infected the flies with Neurospora crassa and monitored survival over 5 days. They repeated with micrococcuss tuteus iv. Conclusion: each of the 2 antimicrobial peptides provided a protective immune response. The different peptides defended against different pathogens. Innate Immunity of Vertebrates Innate defenses that are common in both vertebrates & invertebrates are barrier defenses, phagocytosis, & antimicrobial peptides. Innate defenses unique to vertebrates only are natural killer cells, interferons, & the inflammatory response Barrier Defenses In mammals, epithelial tissues block the entry of many pathogens. They include skin and the mucous membranes lining the digestive, respiratory, urinary, & reproductive tracts Certain cells of the mucous membranes produce mucus, a viscous (hard to flow) fluid that enhances defenses by trapping microbes & other particles. o In the trachea, ciliated epithelial cells sweep mucus & any entrapped microbes, upward, helping prevent infection of the lungs Saliva, tears, & mucous secretions that bathe various exposed epithelia provide a washing action that inhibits colonization by fungi & bacteria Body secretions also provide an environment that’s hostile to many microbes. o Lysozyme in tears, saliva, & mucous secretions destroys the cell walls of susceptible bacteria as they enter the openings around eyes or upper respiratory tract o Microbes in food or water & those swallowed in mucus contend with the acidic environment of the stomach, which kills most of them before they can enter the intestines o Secretions from oil & sweat glands give human skin a pH ranging from 3 – 5, acidic enough to prevent bacteria growth Cellular Innate Defenses Pathogens entering the mammalian body are subject to phagocytosis. Phagocytic cells detect fungal or bacterial components, using several types of receptors. Each Toll-like receptor (TLR) binds to fragments of molecules characteristic of a set of pathogens. The recognized macromolecule is normally absent from the vertebrate body & is an essential component of certain groups of pathogens o Ex: TLR3 (located on the inner surface of vesicles formed by endocytosis) is the sensor for double-stranded RNA (form of nucleic acid common in certain viruses) o Ex: TLR 4 (located on immune cell plasma membranes) recognizes lipopolysaccharide, a type of molecule found on the surface of many bacteria o Ex: TLR5 recoginzes flagellin, the main protein of bacterial flagella After detecting invading pathogens, a phagocytic cell engulfs them, trapping them in a vacuole. The vacuole then fuses with a Lysozyme, leading to destruction of the invaders. It can destruct in two different ways: 1. Gases produced in Lysozyme poison the engulfed pathogens 2. Lysozyme & other enzymes in the lysosome degrade the components of the pathogens The main types of phagocytic cells in the mammalian body are: 1. Neutrophils- circulate in blood; are attracted to signals from infected tissues & then engulf & destroy the infecting pathogens 2. Macrophages (big eaters)- large phagocytic cells; some migrate throughout the body, some reside permanently in organs & tissues where they’re likely to encounter pathogens There are two other types of phagocytic cells that provide additional functions in innate defense: 1. Dendritic cells- stimulate adaptive immunity against pathogens they encounter & engulf; populate tissues (i.e. skin) that contact the environment 2. Eosinophils- have low phagocytic activity but are important in defending against multicellular invaders (i.e. parasitic worms); found beneath mucosal surfaces; Natural killer cells circulate through the body & detect the abnormal array of surface proteins characteristic of some virus-infected & cancerous cells. They DO NOT engulf stricken cells. Instead, they release chemicals that lead to cell death, inhibiting further spread of the virus or cancer Many cellular innate defenses of vertebrates involve the lymphatic system, a network that distributes the fluid, lymph, throughout the body. o Some macrophages reside in lymph nodes, where they engulf pathogen that have flowed from the interstitial fluid into the lymph o Dendritic cells reside outside the lymphatic system but migrate to lymph nodes after interaction with pathogens Within lymph nodes, dendritic cells interact with other immune cells, stimulating adaptive immunity Antimicrobial Peptides & Proteins In mammals, pathogen recognition triggers the production & release of a variety of peptides & proteins that attack pathogens or impede their production. o Some of these defense molecules damage broad groups of pathogens by disrupting membrane integrity. These are common in invertebrates too. o Others (i.e. interferons & complement proteins) are unique to vertebrate immune systems I. Interferons- proteins that provide innate defense by interfering with viral infections. o Virus-infected body cells secrete interferons, which induce nearby uninfected cells to produce substances that inhibit viral reproduction. in this way, interferons limit the cell-to-cell spread of viruses in the body, helping control viral infections (i.e. colds & influenza) o some white blood cells secrete a different type of interferon that helps activate macrophages, enhancing their phagocytic ability II. complement system- infection fighting system that consists of ~30 proteins in the blood plasma aaaaawhich circulate in an inactive state & are activated by substances on the surface of microbes o Activation results in a cascade of biochemical reactions that can lead to lysis of invading cells o Also functions in inflammatory response & adaptive defenses Inflammatory Response Inflammatory response- changes brought about by signaling molecules released upon injury or infection that result in the pain & swelling in the local area where the injury occurred Histamine- an important inflammatory signaling molecule, which is stored in granules (vesicles) of mast cells, found in connective tissue. o Histamine released at sites of damage triggers nearby blood vessels to dilate & become more permeable (“leaky capillaries”) so that fluid containing antimicrobial peptides can more easily enter the tissue cytokines- signaling molecules that are discharged from activated macrophages & neutrophils & enhance the immune response by promoting blood flow to the site of injury. o The increase in local blood supply causes the redness & increased skin temperature Blood-engorged capillaries leak fluid into neighboring tissues, causing swelling During inflammation, cycles of signaling & response transform the site o Activated complement proteins promote further release of histamine, attracting more phagocytic cells that enter injured tissues & carry out additional phagocytosis o Simultaneously, enhanced blood flow to the site helps deliver antimicrobial peptides. This results in an accumulation of pus, a fluid rich in white blood cells, dead pathogens, & cell debris from damaged tissues A minor injury causes a local inflammatory response, but severe tissue damage may lead to a systemic response o Cells in injured tissue often secrete molecules that stimulate the release of additional neutrophils from the bone marrow. Fever is another systemic response. In response to certain pathogens, substances released by activated macrophages cause the body’s thermostat to reset to a higher temperature o Benefits of elevated body temperature caused by fever: enhanced phagocytosis, speeding up of chemical reactions, & accelerated tissue repair Certain bacterial infections can induce an overwhelming systemic inflammatory response, leading to septic shock, a life-threatening condition characterized by very high fever, low blood pressure, & poor blood flow through capillaries Evasion of Innate Immunity by Pathogens Adaptations have evolved in some pathogens that enable them to avoid destruction by phagocytic cells o Ex: streptococcus pneumoniaie, which helped the discovery that DNA can convey genetic info Some bacteria, after being engulfed by a host cell, resist breakdown within lysosomes o Ex: bacteria that causes tuberculosis. Rather than being destroyed within host cells, they grow & reproduce, effectively hidden from the body’s innate immune defenses 43.2 In Adaptive Immunity, Receptors Provide Pathogen-Specific Recognition Only vertebrates have adaptive immunity, which relies on T and B cells, which are types of white blood cells, lymphocytes, which originate in the bone marrow. Some lymphocytes migrate from the bone marrow to the thymus, an organ in the thoracic cavity above the heart. They mature to T cells Lymphocytes that remain & mature in the bone marrow develop as B cells. Some lymphocytes remain in the blood & become natural killer cells active in innate immunity Antigen- any substance that elicits a response from a B or T cell Recognition occurs when a B or T cell binds to an antigen via a protein called an antigen receptor, which is specific enough to bind to just one part of one molecule from a particular pathogen All of the antigen receptors made by a single B or T cell are identical. Infection by a pathogen triggers activation of B & T cells with antigen receptors specific for parts of that antigen. Antigens are foreign & are large macromolecules (i.e. proteins or polysaccharides) o Many antigens protrude from the surface of foreign pathogens o Other antigens (i.e. toxins secreted by bacteria) are released into extracellular fluid Epitope (antigenic determinant)- small, accessible portion of antigen that binds to an antigen receptor o Ex: a group of amino acids in a particular protein A single antigen usually has many different epitopes, each binding a receptor with a different specificity Each B or T cell displays specificity for a particular epitope, enabling it to respond to any pathogen that produces molecules containing the same epitope Antigen Recognition by B Cells & Antibodies Each B cell antigen receptor is a Y-shaped molecule consisting of 2 polypeptide chains: 2 identical heavy chains & 2 identical light chains, with disulfide bridges linking the chains together A transmembrane region near one end of each heavy chain anchors the receptor in the cell’s plasma membrane. A short tail region at the end of the heavy chain extends into the cytoplasm The light & heavy chains each have a constant (C) region where amino acid sequences vary little among receptors of different B cells. The C region includes the cytoplasmic tail & transmembrane region of the heavy chain & all of the disulfide bridges. Within the 2 tips of the Y shape, the light & heavy chains each have a variable (V) region, which has amino acid sequence variation from one B cell to another Together, parts of a heavy-chain V region and a light chain V region form an asymmetrical binding site for an antigen. Each B cell antigen receptor has two identical antigen-binding sites The binding of a B cell antigen receptor to an antigen is an early step in B cell activation, leading eventually to formation of cells that secrete an antibody or immunoglobulin (Ig), a soluble, protein form of the receptor Antibodies have the same Y-shaped receptor as B cell antigen receptors, but they’re secreted, not membrane bound It is the antibodies, not the B cells themselves, that help defend against pathogens The antigen-binding site of a membrane-bound receptor or antibody has a unique shape that provides a lock-and-key fit for a particular epitope Many noncovalent bonds between an epitope & the binding surface provide a stable & specific interaction Differences in amino acid sequences of V regions provide the variation in binding surfaces that enables this highly specific binding B cell antigen receptors & antibodies bind to intact antigens in the blood & lymph Antibodies can bind to antigens on the surface of pathogens or free in body fluids Antigen Recognition by T Cells Structure of T cell antigen receptor: o has 2 different polypeptide chains, an α and a ϐ chain, linked by a disulfide bridge. o Near the base is a transmembrane region that anchors the molecule in the cell’s plasma membrane. o At the outer tip of the molecule, the V regions of the chains together form a single antigen-binding site. o The remainder is made up of C regions. B Cell antigen receptors T cell antigen receptors Bind to epitopes of intact antigens circulating Bind only to fragments of antigens that are in body fluids displayed/ presented on the surface of host cells Major histocompatibility complex (MHC) molecule- host protein that displays the antigen fragment on the cell surface Steps of recognition of protein antigens by T cells 1. Pathogen infects host cell or is taken in by the host cell 2. Inside the host cell, enzymes in the cell cleave the antigen into small peptides, called antigen fragments 3. Each antigen fragment binds to an MHC molecule inside the cell 4. MHC molecule & bound antigen fragment moves to cell surface, resulting in antigen aaaapresentation, the presentation of the antigen fragment in an exposed groove of the MHC protein Antigen presentation advertises the fact that a host cell contains a foreign substance 5. If the cell displaying an antigen fragment encounters a T cell with the right specificity, the antigen receptor on the T cell can bind to the antigen fragment & the MHC molecule This interaction of an MHC molecule, an antigen fragment, & an antigen receptor is necessary for a T cell to participate in an adaptive immune response B Cell & T Cell Development – Four Major Characteristics of adaptive immunity 1. There’s an immense diversity of lymphocytes & receptors, enabling the immune system to detect pathogens never before encountered 2. Adaptive immunity normally has self-tolerance, & the lack of reactivity against one’s own molecules & cells 3. Cell proliferation triggered by activation greatly increases the number of B & T cells specific for an antigen 4. There’s a stronger & more rapid response to an antigen encountered previously due to immunological memory Receptor diversity & self-tolerance arises as a lymphocyte matures Proliferation of cells & the formation of immunological memory occurs later, after a mature lymphocyte encounters & binds to a specific antigen I. Generation of B & T Cell Diversity We can generate such a remarkable diversity in antigen receptors despite the fact that there are only ~20,000 protein-coding genes because of combinations. By combining variable elements, the immune system assembles any different receptors from a much smaller collection of parts to understand the origin of receptor diversity, consider an immunoglobulin (Ig) gene that codes for light-chain of both secreted antibodies & membrane-bound B cell antigen receptors (this process is also very similar for all B & T cell antigen receptor genes) the structure of the Ig genes allows the capacity to generate diversity. A receptor light chain is encoded by 3 gene segments: a V segment, a joining (J) segment, and a C segment. o The V & J segments together encode the V region of the receptor chain. o The light-chain gene contains 1 C segment, 40 different V segments, & 5 different J segments o These alternative copies of V & J segments are arranged within the gene in a series Because a functional gene’s built from 1 copy of each type of segment, the pieces can be combined in 200 different ways Assembling a functional Ig gene requires rearranging the DNA o Recombinase links one light-chain V gene segment to one J gene segment in early B cell development This recombination eliminates the long stretch of DNA between the segments (intron), forming a single exon that's part V & part J o The J & C segments of the RNA transcript will be joined when splicing removes the intervening RNA Recombinase acts randomly, linking any one of the 40 V gene segments to any one of the 5 J segments. o In any given cell, only 1 allele of a light-chain gene & 1 allele of a heavy-chain gene are rearranged o The rearrangements are permanent & are passed on to the daughter cells when the lymphocyte divides After both the light & heavy chain genes have rearranged, antigen receptors can be synthesized. o The rearranged genes are transcribed, & are processed for translation. o After translation, the light & heavy chains assemble together, forming an antigen receptor Each pair of randomly rearrange heavy & light chains results in a different antigen-binding site II. Origin of Self-Tolerance Adaptive immunity distinguishes self from nonself Because antigen receptor genes are randomly rearranged, some immature lymphocytes produce receptors specific for epitopes on organism’s own molecules, but they are eliminated or inactivated. If they were not eliminated, the immune system would not be able to distinguish self from nonself & begin attacking its own proteins, cells, & tissues As lymphocytes mature in the bone marrow or thymus, their antigen receptors are tested for body’s own molecules & are destroyed by apoptosis The remaining self-reactive lymphocytes are made nonfunctional, leaving only the lymphocytes that react to foreign molecules. Since the body normally lacks mature lymphocytes that can react against its own components, the immune system exhibits self-tolerance III. Proliferation of B & T Cells III. Proliferation of a Lymphocyte into a Clone of Cells in Response to Binding an Antigen 1. An antigen is presented to a steady stream of lymphocytes in the lymph nodes until a match is made Clonal selection- an encounter with an antigen selects which lymphocyte will divide to produce a clonal population of thousands of cells specific for a particular epitope 2. A successful match triggers changes in cell number & activity for the lymphocyte to which the antigen has bound to 3. The binding of an antigen receptor to an epitope initiates events that activate the lymphocyte. Once activated, a B or T cell undergoes many cell divisions, resulting in clones 4. The clone cells differentiate a. Some become effector cells, which are short-lived cells that take effect immediately against the antigen & any pathogens producing that antigen. i. B cell effector cells are plasma B cells, which secrete antibodies ii. T cell effector cells are helper T cells & cytotoxic T cells b. The remaining clone cells differentiate into memory cells, long lived cells that can give rise to effector cells if the same antigen’s encountered again Ex: B cell clonal proliferation into a clone cell in response to binding to an antigen. In response to a specific antigen & to immune cell signals, one B cell divides & forms a clone of cells. (The remaining B cells, whose antigen receptors are not specific to the particular antigen don’t respond.) The clone of cells formed by the selected B cell gives rise to memory B cells & antibody-secreting plasma cells. 1. Antigens bind to the antigen receptors of only one specific B cell, whose antigen receptor matches the particular epitope 2. The selected B cell proliferates, forming a clone of identical cells bearing receptors for the antigen 3. Some daughter cells develop into long-lived memory cells that can respond rapidly upon subsequent exposure to the same antigen, while other daughter cells develop into short-lived plasma cells that secrete antibodies for the antigen IV. Immunological Memory Immunological memory’s responsible for the long-term protection that a prior infection or vaccination provides against many diseases Prior exposure to an antigen alters the speed, strength, & duration of the immune response Primary immune response- production of effector cells from a clone of lymphocytes during the first time exposure to an antigen o Peaks ~10 – 17 days after the initial exposure. During this time, selected B & T cells give rise to their effector forms Secondary immune response- if an individual is exposed again to the same antigen, the response is faster (peaks 2 – 7 days after exposure), of greater magnitude, & more prolonged o Because selected B cells give rise to antibody secreting effector cells, measuring the concentrations of specific antibodies in blood over time distinguishes the 1st & 2nd immune responses 43.3 Adaptive Immunity Defends Against Infection of Body Fluids & Body Cells This section discusses how lymphocytes help fight infections & minimize damage by pathogens Both Humoral immune response Cell mediated immune Include a primary & secondary response occurs in blood & lymph; specialized T cells destroy antibodies help neutralize or immune response (which is enabled with memory cells) eliminate toxins & infected host cells pathogens in the blood & lymph produced by activity of B lymphocytes Helper T Cells: A Response to Nearly All Antigens Helper T cell- triggers both humoral & cell-mediated immune responses; they themselves don’t carry out those responses; rather, they send signals that initiate production of antibodies that neutralize pathogens & activate T cells that kill infected cells Two requirements must be met for a helper T to activate adaptive immune responses 1. Foreign molecule must be present that can bind specifically to the antigen receptor of the T cell 2. This antigen must be displayed on the surface of an antigen-presenting cell (APC), which can be a dendritic cell, macrophage, or B cell When host cells are infected, they also display antigens on their surface. Antigen-presenting cells have class I & class II MHC molecules o The class II molecules provide a molecular signature by which an APC is recognized A helper T & the APC displaying its specific epitope have a complex interaction 1. The antigen receptors on the surface of the helper T bind to the antigen fragment & to the class II MHC molecule displaying that fragment on the APC. At the same time, an accessory protein, CD4, on the helper T surface binds to MHC II, helping to keep the cells joined 2. When the APC & helper T interact, signals in the form of interleukins (a type of cytokines) secreted from a dendritic cell act in combo with the antigen to stimulate the helper T, causing it to produce its own set of interleukins. The different types of APCs interact with helper Ts in distinct ways. o If the APC is a dendritic cell or macrophage: 1. A helper T is activated 2. Helper T proliferates, forming a clone of activated helper Ts 3. B cells present antigens to already activated helper Ts, which activates the B cells themselves Cytotoxic T Cells: A Response to Infected Cells In cell-mediated immune response, the effector cells are cytotoxic T cells, which use their toxic gene products to kill infected cells To become active, they require signaling molecules from helper T & interaction with a host cell that presents an antigen. Once activated, cytotoxic T can eliminate cells that are infected by intracellular pathogens 1. Fragments of foreign proteins produced in infect host cells associate with class I MHC molecules & are displayed on the cell surface, where they can be recognized by cytotoxic Ts. 2. Cytotoxic Ts bind with accessory protein CD8, which helps keep the 2 cells in contact while the T cell is activated 3. The targeted destruction of an infected host cell by cytotoxic T involves the secretion of proteins that disrupt membrane integrity & trigger apoptosis. o The death of the infected cell deprives the pathogen of a place to reproduce & exposes cell contents to circulating antibodies, which mark them for disposal 4. After destroying an infected cell, cytotoxic T can move on & kill other cells infected with the same pathogen B Cells & Antibodies: A Response to Extracellular Pathogens Activation of B Cells Activation of humoral immune response involves B cells & helper T cells & proteins on the surface of pathogens 1. B cell activation by an antigen is aided by cytokines (interleukin 1) secreted from helper Ts that have encountered the same antigen. 2. Stimulated by both an antigen & interleukin 1, the B cell proliferates & differentiates into memory B & antibody-secreting effector plasma cells The pathway for antigen processing & display in B cells differs from that in other APCs. o A macrophage or dendritic cell can present fragments from a wide variety of protein antigens o B cell presents only the antigen to which is specifically binds 1. When an antigen first binds to receptors on the surface of a B cell, B cell takes in a few foreign molecules by receptor-mediated endocyotsis. 2. Class II MHC protein of the B cell then presents an antigen fragment to a helper T. This direct cell-to-cell contact is critical to B cell activation. An activated B cell gives rise to thousands of identical plasma cells, which stop expressing a membrane-bound antigen receptor & begin producing & secreting antibodies Most antigens recognized by B cells contain multiple epitopes, so an exposure to a single antigen normally activates a variety of B cells, with different plasma cells producing antibodies directed against different epitopes on the common antigen Antibody Function Antibodies don’t kill pathogens. Instead, when they bind to antigens, they mark pathogens in several ways for inactivation or destruction: 1. Neutralization- antibodies bind to viral surface proteins. o The bound antibodies prevent infection of a host cell, neutralizing the virus. o Antibodies sometimes bind to toxins released in body fluids, preventing the toxins from entering the body 2. Opsonization- antibodies bound to antigens on bacteria present a readily recognized structure for macrophages or neutrophils & increase phagocytosis o Because each antibody has 2 antigen-binding sites, antibodies sometimes also facilitate phagocytosis by linking foreign substances into aggregrates 3. Antibodies sometimes work together with proteins of the complement system to dispose of pathogens o Binding of a complement protein to an antigen-antibody complex on a foreign cell or enveloped virus triggers a cascade in which each protein of the complement system activates the next protein Ultimately, activated complement proteins generate a membrane attack complex that forms a pore in the membrane of the foreign cell. Ions & water rush into the cell, causing it to lyse. This cascade of complement protein activity results in lysis of foreign cells & produces factors that promote inflammation or stimulate phagocytosis When antibodies facilitate phagocytosis, they also help fine-tune the humoral immune response o Phagocytosis enables macrophages & dendritic cells to present antigens to & stimulate helper Ts, which then stimulate B cells whose antibodies contribute to phagocytosis This is a positive feedback between innate & adaptive immunity Antibodies can also bring about the death of infected body cells. o When a virus uses a cell’s biosynthetic machinery to produce viral proteins, these viral products can appear on the cell surface o If antibodies specific for epitopes on these viral proteins bind to the exposed proteins, the presence of bound antibody at the cell surface can recruit a natural killer cell, which then releases proteins that cause the infected cell to undergo apoptosis B cells can express 5 different forms of antibodies or immunoglobulin (Ig) For a given B cell, each form or class has an identical antigen-binding specificity, but a distinct heavy-chain C region 1. IgD- the only B cell antigen receptor that's membrane bound. The other 4 classes consist of soluble antibodies 2. IgM- first class of soluble antibody produced 3. IgG- follows next, is the most abundant antibody in blood 4. IgA 5. IgE Summary of the Humoral & Cell-Mediated Immune Responses Both responses can include Io & IIo responses Memory cells of each type – helper T & B & cytotoxic T – enable the IIo response o Ex: when body fluids are reinfected by a pathogen encountered previously, memory B & T initiate a IIo humoral response Active & Passive Immunization Active immunity- defenses that arises when a pathogen infects the body & prompts a Io or IIo response Passive immunity- immunity that results when the IgG antibodies in the blood of a pregnant female cross the placenta to her fetus. The transferred antibodies can immediately react with any pathogens for which they’re specific; the antibodies provided by the mother guard against pathogens that have never infected the newborn o Because passive immunity doesn’t involve the recipient’s B & T cells, it persists only as long as the transferred antibodies last After giving birth, a nursing mother continues to transfer protection against disease to her infant. IgA antibodies present in breast milk provide additional passive immunity to the infant’s digestive tract while the infant’s immune system develops. o Later on, IgA functions in active immunity: IgA antibodies secreted in tears, saliva, & mucus protect the mucous membranes Active & passive immunity can be introduced artificially. Immunization- allows active immunity to develop from intro of antigens into the body In 1796, Edward Jenner used cowpox virus to induce adaptive immunity against the related smallpox Many agents of antigens (i.e. inactivated bacterial toxins, killed pathogens, part of pathogens, weakened pathogens that don’t cause illness, & genes encoding microbial proteins) are used today to make vaccines. They induce a primary immune response & immunological memory, so an encounter with the pathogen from which the vaccine was derived triggers a rapid & strong secondary immune response) Antibodies as Tools in Research, Diagnosis, & Therapy Some antibody tools are polyclonal: they’re products of many different clones of plasma cells, each specific for a different epitope. o Antibodies that an animal produces after exposure to a microbial antigen are polyclonal Monoclonal antibodies- prepared from a single clone of B cells grown in culture; identical & specific for the same epitope on an antigen Medical diagnosis & treatment using monoclonal antibodies o Ex: home pregnancy kits use monoclonal antibodies to detect human chorionic gonadtrophin (hCG), which is produced as soon as an embryo implants in the uterus. The presence of this hormone in her urine indicates for a very early stage of pregnancy o Ex: for therapy to treat human diseases, researchers use mouse B cell clones to identify antibodies specific for an epitope on diseased cells. Then, the mouse antibody genes are altered to code for antibodies that appear less foreign to the human adaptive immune defenses. Then, scientists use the humanized genes to produce large amounts of antibody for injecting into patients Immune rejection Cells from another person can be recognized as foreign & attacked by immune defenses. This is the expected reaction of a healthy immune system exposed to foreign antigens 1. Blood Groups To avoid a blood transfusion being recognized as foreign by the recipient’s immune system, the blood groups of the donor needs to have the same type carbohydrate on the surface of their RBCs (i.e. both A & B carbohydrates are found on type AB RBCs & neither carbohydrate is found on O) Ex: immune response of male with type A blood o Certain bacteria normally present in the body have epitopes very similar to the A & B carbohydrates. o By responding to the bacterial epitope similar to the B carbohydrate, a person with type A blood makes antibodies that’ll react with the type B carbohydrate o No antibodies are made against the bacterial epitope similar to the type A carbohydrate because lymphocytes reactive with the body’s own molecules are inactivated or eliminated during development o If he receives a transfusion of type B blood, his anti-B antibodies cause an immediate transfusion reaction. The transfused RBCs undergo lysis, which can damage health 2. Tissue & Organ (Graft) Transplants MHC molecules stimulate the immune response that leads to rejection Each vertebrate species has many alleles for each MHC gene, enabling presentation of antigen fragments that vary in shape & electrical charge, guaranteeing that no two people have exactly the same set, so in vast majority of graft & transplant recipients To minimize rejection, physicians use donor tissue bearing MHC molecules that match those of the recipients very closely & the recipient needs to take meds that suppress immune responses Bone marrow transplants are used to treat leukemia & hematological (blood cell) diseases. Transplants of bone marrow can cause immune reaction. o Before receiving transplanted bone marrow, the recipient’s treated with radiation to eliminate their own blood marrow cells, destroying the source of the normal cells This treatment obliterates the recipient’s immune system, leaving little chance of graft rejection But, lymphocytes in the donated marrow may react against the recipient’s o This graft vs host reaction is limited if the MHC molecules of the donor & recipient are well matched 43.4 Disruptions in Immune System Function can Elicit or Exacerbate Disease Exaggerated, Self-Directed, & Diminished Immune Responses Allergies Allergies are exaggerated (hypersensitive) responses to certain allergens, specific antigens. The most common allergies involve antibodies of the IgE class o Ex: hay fever occurs when plasma cells secrete IgE antibodies specific for antigens on the surface of pollen grains. Some IgE antibodies attach by their base to mast cells in connective tissues Pollen grains that enter the body attach to the antigen-binding sites of those IgE bodies. This attachment links adjacent IgE molecules, inducing the mast cell to release histamine & other inflammatory chemicals from granules (vesicles) Antihistamines diminish allergy symptoms & inflammation by blocking receptors for histamine Anaphylactic shock- acute allergic response in which the whole-body reacts in a life-threatening way after exposure to an allergen; develops when widespread release of mast cell contents triggers abrupt dilation of peripheral blood vessels, causing a huge drop in blood pressure & the constriction of bronchioles People with severe hypersensitivities carry syringes containing epinephrine, which counteracts this allergic response Autoimmune diseases Autoimmune disease- caused by a person’s own immune system active against particular molecules of their own body There are many forms of such a loss of self-tolerance: 1. Lupus- immune system generates antibodies against histones & DNA released by the normal breakdown of body cells. 2. Rheumatoid arthritis- leads to damage & painful inflammation of the cartilage & bone of joints 3. Type 1 diabetes mellitus- insulin producing beta cells of the pancreas are the targets of autoimmune cytotoxic cells 4. Multiple sclerosis- most common; T cells infiltrate the central nervous system, resulting in destruction of the myelin sheath that surrounds parts of many neurons, leading to muscle paralysis through a disruption in neuron function Exertion, Stress, & the Immune System Exercise to the point of exhaustion leads to more frequent infections & to more severe symptoms o On average, marathon runners get sick less than their more sedentary peers during training, but have a marked increase in illness in the period immediately following the race Psychological stress can disrupt immune system regulation by altering the interplay of the hormonal, nervous, and immune system Immunodeficiency Diseases Immunodeficiency- disorder in which an immune system response to antigens is defective or absent 1. Inborn immunodeficiency results from a genetic or developmental defect in immune system, , leading in impairment of innate and/or adaptive defenses o Result from defects in development of various immune system cells or defects in production of specific proteins (i.e. antibodies or proteins of complement system) o In severe immunodeficiency (SCID), functional lymphocytes are rare or absent. Lacking an adaptive immune response, SCID patients are susceptible to infections that can cause death in infancy Treatments: bone marrow & stem cell transplantation 2. Acquired immunodeficiency develops later in life following exposure to chemical or biological agents o Drugs used to fight autoimmune diseases or prevent transplant rejection suppress the immune system, leading to immunodeficient state o Ex: Acquired immunodeficiency syndrome (AIDS), which is caused by human immunodeficiency virus (HIV) Evolutionary Adaptations of Pathogens that Underlie Immune System Avoidance Antigenic variation Pathogen alters how it appears to the immune system o Immunological memory is a record of the foreign epitopes previously encountered If the pathogen that expressed those epitopes doesn’t anymore, it can reinfect or remain in a host without triggering the rapid response of memory cells Antigenic variation- such changes in epitope expression; regular events for viruses & parasites o Ex: sleeping sickness periodically switches at random among 1000 different versions of the protein found over its entire surface, so it can persist in the body without facing an effective adaptive immune response Antigenic variation is the major reason why influenza virus remains a health problem o As it replicates in one human host cell after another, the human influenza virus mutates o Because any change lessens recognition by the immune system provides a selective advantage, so the virus accumulates with alterations Human virus occasionally exchanges genes with influenza viruses that infect domesticated animals. o When this happens, influenza can take on such a radically different appearance that none of the memory cells in humans can recognize the new strain o Ex: H1N1 = flu viruses from pigs + humans + birds Latency/ Lysogenic Cycle After infecting a host, some viruses enter a largely inactive state called latency Because such dormant viruses stop making most viral proteins & produce no free virus particles, they don’t trigger an adaptive immune response But, the viral genome persists in the nuclei of infected cells, either as separate small DNA molecule Latency persists until conditions arise that are favorable for viral transmission (i.e. when host’s infected by another pathogen) Ex: herpes establish themselves in human sensory neurons. o Because sensory neurons express few MHC I molecules, the infected cells are inefficient at presenting viral antigens to circulating lymphocytes o Stimuli like fever, stress, or periods reactivate the virus to reproduce & infect surrounding epithelial tisses Attack on the Immune System: HIV HIV, the pathogen that causes AIDS, escapes & attacks the adaptive immune response Once HIV is introduced into the body, it infects helper Ts with high efficiency. To infect helper Ts, the virus binds specifically to the CD4 accessory protein and some cell types that have low levels of CD4 (i.e. macrophages & brain cells) In the cell, the HIV RNA genome is reverse-transcribed, & the product DNA’s integrated into the host cell’s genome. In this form, the viral genome can direct production of new virus particles Although the body responds to HIV with an immune response that can eliminate most viral infections, some HIV escapes HIV persists because of antigenic variation. It mutates at a very high rate during replication. Altered proteins on the surface of some mutated viruses reduce interaction with antibodies & cytotoxic T cells. o These viruses survive, proliferate, & mutate further; the virus evolves inside the body Latency also helps HIV persistence. o When the viral DNA integrates into the chromosome of a host cell but doesn’t produce new virus proteins, it’s shielded from the immune system of the host cell. o This inactive viral DNA is protected from antiviral agents used against HIV because they attack only actively replicating viruses Over time, an untreated HIV infection abolishes the adaptive immune response Viral reproduction & cell death triggered by the virus lead to loss of helper Ts, impairing both humoral & cell0mediated immune responses. This results in progression to AIDS, characterized by a susceptibility to infections & cancers that a healthy immune system would usually defeat o These opportunistic diseases & nerve damage & body wasting are the primary causes of death in AIDS patients, not HIV itself There is currently no cure to AIDS. Mutations that occur in each round of viral reproduction can generate strains of HIV that are drug resistant. The impact of such viral drug resistance can be reduced by the use of a combo of drugs. o But, the appearance of strains resistant to multiple drugs can reduce the effectiveness of such multidrugs cocktails Transmission of HIV requires the transfer of virus particles or infected cells from person to person via body fluids. People infected with HIV can transmit the disease in the first few weeks of infection, before they express HIV-specific antibodies that can be detected in a blood test Cancer & Immunity When adaptive immunity is inactivated, the frequency of certain cancers increases dramatically If the immune system recognizes only nonself, it should fail to recognize the uncontrolled growth of self cells that’s the hallmark of cancer. Viruses are involved in 15 – 20% of all human cancers Because immune system can recognize viral proteins as foreign, it can act as a defense against viruses that can cause cancer & against cancer cells that harbor viruses o Kaposi’s sarcoma herpesvirus o Hepatitis B virus, can trigger liver cancer. First vaccine to help prevent specific human cancer Vaccinating Against Cervical Cancer Human papillomavirus (HPV) can cause cervical cancer 2 particular types of HPV from patients of cervical cancer were isolated, copied, leading to the development