Designing a Separations Process When VLE Data is not
advertisement

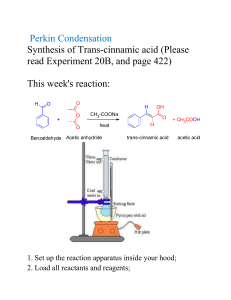
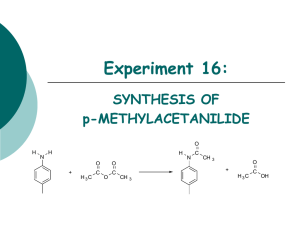
Designing a Separations Process Without VLE Data by Thomas Schafer - Koch Modular Process Systems, LLC This presentation utilizes as it’s example a problem presented to KMPS by a pharmaceutical client who was incinerating a valuable solvent stream. Components to be separated - Toluene from Acetic Anhydride Problem Definition - Customer Objectives & Physical Properties PROCESS OBJECTIVES COMPONENT Table 1 FEED Toluene , wt % 40 Acetic Anhydride, wt % 60 Recovered Toluene Product Recovered Acetic Anhydride Product 99.99% < 100 ppm acetic anhydride or acetic acid 1% 99% PHYSICAL PROPERTIES PROPERTY Table 2 Molecular Weight Boiling Point, at 760 torr, oC Antoine Constants: A LN(VP)=A-B/(T+C): B VP(=)mmHg, T(=)K: C Water Solubility, g/100 g H2O Liquid Density, g/cm3 Melting Point, oC COMPONENT Toluene Acetic Anhydride 92.0 110.8 16.0137 3,096.52 -53.67 0.05 0.866 -95.0 102.0 139.6 16.3982 3,287.56 -75.11 12.0 1.082 -73.0 Approaches That Should Be Considered When VLE Data is Not Available Engineers may choose from the following alternatives: 1. Assume the compounds form an ideal solution, which means vapor pressure vs. temperature data can be used to predict VLE. This is generally acceptable when the compounds are closely related, such as members of a homologous series. Examples include linear alcohols, paraffinic hydrocarbons, aliphatic substituted benzene (benzene, toluene, xylene), polymeric glycols. 2. Find VLE data for an analogous system, one that contains one of the compounds of the pair. The 2nd compound should be closely related to the other compound of the pair, containing the same or similar structure and functional groups. An example of this technique would be to use liquid activity coefficients of benzene and ethanol to predict VLE for benzene and propanol. 3. Develop VLE data for key pairs of components. Set up a VLE apparatus to test each component pair. Data developed this way can be regressed to provide interaction coefficients which can then be used in a process simulator to explore a range of design alternatives. Analogous Systems Data The literature was then searched for VLE data for an analogous system. Data for benzene-acetic anhydride (Figure 1A) and cyclohexane-acetic anhydride (Figure 1B) were found in Dechema. These data clearly indicate non-ideality. Figure 1A Figure 1B Conclusions Drawn From Analogous System Data After analyzing the analogous system data, an engineer should expect that the tolueneacetic anhydride system will exhibit similar but more severe non-ideality because toluene boils closer to acetic anhydride than either cyclohexane or benzene. Two initial predictions of the VLE curve for toluene-acetic anhydride were made by using the benzene-acetic anhydride and cyclohexane-acetic anhydride NRTL coefficients, with toluene vapor pressure data. The predicted curves are plotted in Figure 2. The data indicate that azeotropic behavior is probable. Figure 2 Since the plotted data showed significant non-ideality it was decided that generating VLE data was the preferred alternative. VLE Apparatus To generate the VLE data, a simple, inexpensive apparatus was constructed of glass and polytetrafluoroethylene components, similar to the design shown in Figure 3. It is critical that the apparatus yield exactly one theoretical stage. Figure 3 Calibration of VLE Appartus with Known System After assembly of the apparatus shown in Figure 3, a known system was checked to ensure that the apparatus will yield exactly one theoretical stage. The known system should boil in a similar temperature range to the experimental system. Internal condensation must be avoided as it can result in up to two theoretical stages in the test apparatus. Data for ethanol-water was then compared to literature data as shown in Table 3. As can be seen, the test system results in almost exactly one theoretical stage. ETHANOL-WATER SYSTEM EXPERIMENTAL REFERENCE EXPERIMENTAL Ethanol in Ethanol in Vapor Ethanol in Vapor K Value Liquid m.f. m.f. m.f. 0.1414 0.1598 0.2414 0.5095 0.5049 0.5627 0.4911 0.5055 0.5466 Table 3 3.60 3.16 2.33 REFERENCE K Value 3.47 3.16 2.26 Experimental Toluene-Acetic Anhydride Data Using the calibrated experimental setup, VLE data for toluene-acetic anhydride was generated over a range of compositions. The data is shown in Table 4. More data was collected at the toluene rich end of the curve due to predictions from the analogous systems that there may be an azeotrope or possibly an asymptote in the VLE curve. TOLUENE-ACETIC ANHYDRIDE SYSTEM MOLE FRACTIONS LIQUID Temp. (oC) Acetic Anhydride Toluene 107.5 107.5 108.0 108.1 108.5 111.0 0.02835 0.05673 0.08502 0.12518 0.19692 0.37074 0.97165 0.94327 0.91498 0.87482 0.80308 0.62926 VAPOR Acetic Toluene Anhydride Relative Volatility 0.03010 0.05278 0.07973 0.10000 0.13114 0.18605 0.94030 1.07926 1.07253 1.28777 1.62467 2.57750 0.96990 0.94722 0.92027 0.90000 0.86886 0.81395 Table 4 The data was then regressed and NRTL coefficients were derived. A smooth curve was then developed for the system using the NRTL coefficients as shown in Figure 4. Figure 4 Azeotrope Found A minimum boiling azeotrope was calculated at 96 mole% (95.6 wt%) toluene and 4 mole% acetic anhydride. Figure 5 is an enlarged plot of the toluene-acetic anhydride VLE curve in the range of 95-100 mole% toluene. Figure 5 Because the components form an azeotrope, it is not possible using simple distillation to separate the components into pure acetic anhydride and pure toluene. Process Design Given the feed composition, a single distillation column is adequate to recover relatively pure acetic anhydride as a bottoms product and a mixture which approaches the azeotropic composition as a distillate. Approximately 93% of the acetic anhydride was recovered in one pass through this distillation column. Some design parameters for the distillation column are: Theoretical stages Packing Type Packed Height Reflux Ratio 19 Flexipac® 2Y 33 ft. 1.4 Distillate Composition Bottoms Composition 91 wt% Toluene 99 wt% Acetic Anhydride The toluene in the distillate was recovered by water extraction to remove the small amount of acetic anhydride. Figure 6 is a photo of a modular process system that was built to perform the separation described. The resulting process recovers 92% of the acetic anhydride and 99% of the toluene from a stream that was previously incinerated. Figure 6 Modular Separation System for Recovery of Toluene & Acetic Anhydride