SUPPLEMENTARY MATERIAL Apoptogenic effects of β
advertisement

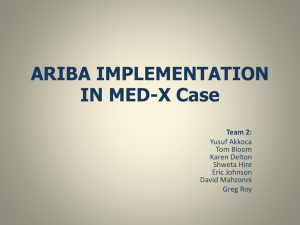
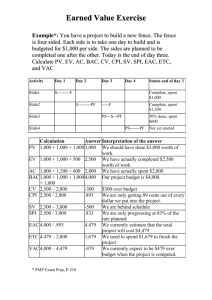
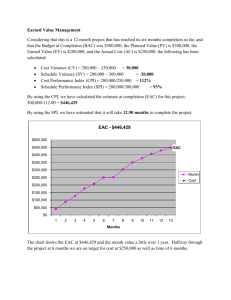
SUPPLEMENTARY MATERIAL Apoptogenic effects of β-sitosterol glucoside from Castanopsis indica leaves Narayan Dolaia, Ashish Kumarb, Aminul Islamc & Pallab K. Haldara,* a Department of Pharmaceutical Technology, Jadavpur University, Kolkata 700032, India. b Chemistry Division, CSIR-Indian Institute of Chemical Biology, Kolkata- 700032, India c Natreon Inc, Salt Lake City, Kolkata 700091, India. Abstract β-sitosterol glucoside (BSSG) is a natural biologically active substance isolated from the Castanopsis indica leaves. This study explored the apoptogenic mechanistic studies of BSSG against Ehrlich ascites carcinoma (EAC) treated mice through morphological study, comet assay, flow cytometry (FACS) and western blotting assay method. AO/EB staining and FACS analysis showed that BSSG possessed apoptosis induction activities on EAC cells. Dose dependent induction of DNA damage was observed after BSSG treatment. Increase the expression of apoptotic protein p53 and p21 in EAC, multiple downstream factors contributing to apoptosis pathway. The increase of caspase-9, caspase-3 activities revealed that caspase was a key mediator of the apoptotic pathway induced by BSSG, and up-regulation of Bax and downregulation of anti-apoptotic protein Bcl-2 resulted in the decrease of Bcl-2/Bax ratio. Owing to the combination of significant antitumor activity by inducing apoptosis, BSSG holds the promise of being an interesting chemo-preventive agent active in cancer therapy. Keywords: Castanopsis indica; BSSG; Apoptosis; Comet assay; Chemoprevention. ______________________________________________________________________________ *Corresponding authors. Email: pallab_haldar@rediffmail.com 1. Experimental 1.1 Plant material C. indica leaves were collected from the middle hill region of Sikkim in during September 2010 and authenticated by the Botanical Survey of India, Gangtok, India. A voucher specimen (No. SHRC-5/5/2010/Tech.276) has been deposited in Phytotherapy and Pharmacology Research Laboratory of Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India for future reference. 1.2. Extraction and isolation of compounds First the collected leaves were shade dried at room temperature for 7 days and then powdered in a mechanical grinder. Next, the powdered plant material (1 kg) was successively extracted with petroleum ether (60–80 ºC) followed by methanol using Soxhlet extraction apparatus. Then solvent was completely evaporated under reduced pressure, in vacuo at 40 °C to render the petroleum extract (40.0 g; yield 4% w/w) and methanol extract (120.2 g; yield 12.02 % w/w). After that, methanol extract (100 g) was fractionated with hydrated n-butanol and dried in rotary evaporator. The n-butanol fraction (16 g) was repeatedly chromatographed over silica gel (Mesh 100–200) that was packed using wet packing method in petroleum ether. The column was initially eluted with petroleum ether (60–80 ºC) followed by gradient elution with the mixture of petroleum ether with an increasing amount of chloroform and methanol. These elutes were collected in a series of test tubes (more than 150 tubes) with 20 ml in each fraction. The elutes of similar behaviour (similar Rf values) were pooled together and allowed to stand at room temperature. TLC plate [silica gel 60 F254 (Merck)] was used to monitor the elutes and fractions were visualized by spraying with Liebermann–Burchard reagent followed by heating at 120 ºC and also visualized by iodine spray. Finally eluate Compound A yielded a single spot when subjected to TLC using solvent systems chloroform: methanol (4:1). Compound A yellowish amorphous powder (50 mg) with melting point (275.6–277.4 °C) was further subjected to Proton NMR (300 MHz) and Carbon‐13 NMR (75 MHz) to ascertain the chemical structure. 1.3. Animals Swiss albino mice of about 8 weeks of age with an average body weight of 20–25 g were used for the experiment. The mice were grouped and housed in poly acrylic cages (38 cm × 23 cm × 10 cm) with not more than six animals per cage. The animals were maintained under standard laboratory conditions (temperature 25–30 °C and 55–60% relative humidity with dark/light cycle 14/10 h) and were allowed to free access of standard dry pellet diet and water ad libitum. The mice were acclimatized to laboratory conditions for 7 days before commencement of the experiment. All the described procedures were reviewed and approved by Jadavpur University Animal Ethics Committee. 1.4. Cell culture EAC cells were obtained from Chittaranjan National Cancer Institute, Kolkata, India. Ascitic fluid was drawn out from EAC tumor bearing mouse at the log phase (days 7–8 of tumor bearing) of the tumor cells. The EAC cells were maintained in vivo in Swiss albino mice by intraperitoneal transplantation of 2 × 106 cells per mouse after every 10 days and it is used for present experiment. 1.5. Cell viability assay Cell viability was determined by using the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium (MTT) assay (Bhattacharya, Prasanna, & Haldar, 2011). Briefly, 0.1 ml of cell suspension was seeded in 96-well plates (Greiner, Frickenhausen, Germany) with a seeding density of 1 × 104 cells/well. The cells were treated with different concentrations of BSSG (25, 50, 100, 150 and 200 μg/ml) and incubated for 24 h at 37 °C, 5% CO2 with 98% relative humidity. After incubation, 20 μl of MTT (5 mg/ml) in phosphate buffered saline (PBS) were added to each well and the plates were incubated for 4 h at 37 °C. The colored formazan crystals which were produced from MTT were dissolved in 150 μl of dimethyl sulfoxide (DMSO) and the absorbance was measured at a wavelength of 570 nm by ELISA plate reader. All determinations were carried out in triplicate. Concentrations of BSSG showing 50% reduction in cell viability (ie, IC50 values) were then calculated. 1.6. Tumor model Swiss albino mice (20–25 g) were divided in to three groups (n=12). Group–I served as EAC control and Group II–III served as treated group. All the animals in each group were being injected EAC cells (2×106 cells/mouse, i.p.). This was marked as day ‘0’. After 24 h, EAC transplanted Group-II and III were being injected BSSG (50 and 100 mg/kg b.w, i.p.) once daily for 14 consecutive days (Dolai et al., 2012b). After administration of last dose, the mice were kept fasting for 18 h and then sacrificed for collection of EAC cells to check the apoptogenic properties of BSSG. 1.7. Acridine orange (AO) and ethidium bromide (EB) double staining DNA-binding dyes AO and EB (Sigma Aldrich, USA) were used for the morphological detection of apoptotic and necrotic cells (Attari, Sepehri, Delphi, & Goliaei, 2009). EAC cells (1×106) were collected from sacrificed mice. The cells were detached, washed by cold PBS and then stained with a mixture of AO (100 μg/ml) and EB (100 μg/ml) at room temperature for 5 min. The stained cells were observed by a fluorescence microscope (Leica DM 3000, Germany) at 40X magnifications. The cells were divided into four categories as follows: living cells (normal green nucleus), early apoptotic (bright green nucleus with condensed or fragmented chromatin), late apoptotic (orange-stained nuclei with chromatin condensation or fragmentation) and necrotic cells (uniformly orange-stained cell nuclei). 1.8. DNA damage by comet assay The extent of DNA was quantified by alkaline single cell gel electrophoresis (SCGE), also known as comet assay. Briefly, cells suspended in 0.5% (w/v) low melting agarose were layered over a frosted microscopic slide coated with a layer of 1% normal melting agarose. The slides were left in a lysing solution overnight at 4 °C. Electrophoresis was carried out for 30 min (280 mA, 20 V) at 4 °C. The slides were washed thrice with neutralizing buffer (Tris 0.4 M, pH 7.5), stained with EB, examined under a fluorescence microscope (Leica DM3000, Germany) and subjected to image analysis using CometScore software (Das, Mandal, & Chaudhuri, 2014). 1.9. Flow cytometric analysis (FACS) Assay was performed using procedure described in the reagent-kit purchased from BD Biosciences and protocol was followed by manufacturer’s instruction. To distinguish between apoptosis and necrosis, in a double labeling system, EAC cells from untreated or BSSG treated tumor-bearing mice were washed twice with cold PBS and then re-suspended in 1X binding buffer at a concentration of 1×106 cells/ml. Then 100 µl of the cell suspension was transferred to the 5 ml culture tube. After that, added 25 µl of Annexin V-FITC and/or propidium iodide (PI) solution to the cell suspension. The cell were gently mixed by vortexing and incubated for 15 min at 37 °C. Then 400 µl of 1X binding buffer was added to each tube and analyzed by FACS within one hour using flowcytometer (BD LSRFortessaTM Cell analyzer, USA). 1.10. Apoptotic protein expression by Western blotting EAC lysate was loaded into a 10% SDS-polyacrylamide gel. After electrophoresis, the gel was transferred to nitrocellulose membrane and blocked with 5% Bovin serum albumin (BSA) in 1X tris buffer saline (TBS). The membrane was then incubated with specific primary antibody of p53, p21, Bax, Bcl-2, cleaved caspase-3, cleaved caspase-9 and β-actin (1:1000) for overnight at 4 °C. The protein of interest was visualized by treating with alkaline phosphatase (ALP) conjugated specific secondary antibody. The target protein band was then visualized using bromochloroindolyl phosphate (BCIP) and nitrobluetetrazolium (NBT) substrates. Equal loading of protein in each lane was established by β-actin antibody probing (Chatterjee et al., 2013). References Attari, F., Sepehri, H., Delphi, L., & Goliaei, B. (2009). Apoptotic and necrotic effects of pectic acid on rat pituitary GH3/B6 tumor cells. Iranian Biomedicine Journal, 13, 229–236. Bhattacharya, S., Prasanna, A., & Haldar, P.K. (2011). Evaluation of antiproliferative activity of Trichosanthes dioica root against Ehrlich ascites carcinoma cells. Academic Journal of Cancer Research, 4, 38–42. Chatterjee, S., Biswas, G., Chandra, S., Saha, G.K., & Acharya, K. (2013). Apoptogenic effects of Tricholoma giganteum on Ehrlich’s ascites carcinoma cell. Bioprocess and Biosystems Engineering, 36, 101–107. Das, B., Mandal, S., & Chaudhuri, K. (2014). Role of arginine, a component of aqueous garlic extract, in remediation of sodium arsenite induced toxicity in A375 cells. Toxicological Research, 3, 191–196. Dolai, N., Karmakar, I., Kumar, R.B.S., Bala, A., Majumder, U.K., & Haldar, P.K. (2012). Antitumor potential of Castanopsis indica (Roxb. ex. Lindl.) A. DC. leaf extract against Ehrlich’s ascites carcinoma cell. Indian Journal of Experimental Biology, 50, 359–365. Figure captions Figure S1. Structure of β-sitosterol glucoside Figure S2. BSSG induces apoptotic morphological changes on EAC cells. (a) EAC control group, (b) BSSG 50 mg/kg and (c) BSSG 100 mg/kg. The cells were stained with AO/EB (100 μg/ml). Blue arrows next to "L" point to live cells; Yellow arrows next to "A" indicate apoptotic cells; and Red arrows next to "N" indicate necrotic cells (magnification at 40X). Figure S3. The DNA damage was measured by comet assay after BSSG treatment on EAC cells. (a) EAC control group, (b) BSSG 50 mg/kg and (c) BSSG 100 mg/kg. (d) The extent of DNA damage was expressed in terms of comet % tail length. Data are the mean ± SD from three replicate measurements. Treated groups vs. EAC control group, **p < 0.01. Figure S1. Figure S2. Figure S3.