Notes
advertisement
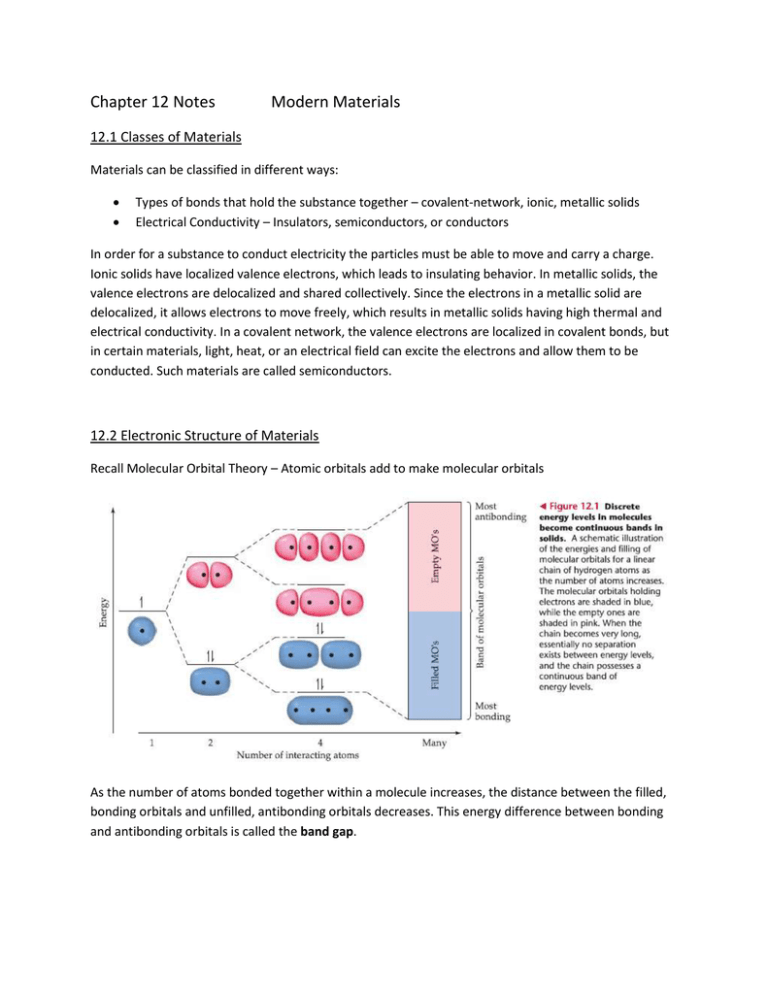
Chapter 12 Notes Modern Materials 12.1 Classes of Materials Materials can be classified in different ways: Types of bonds that hold the substance together – covalent-network, ionic, metallic solids Electrical Conductivity – Insulators, semiconductors, or conductors In order for a substance to conduct electricity the particles must be able to move and carry a charge. Ionic solids have localized valence electrons, which leads to insulating behavior. In metallic solids, the valence electrons are delocalized and shared collectively. Since the electrons in a metallic solid are delocalized, it allows electrons to move freely, which results in metallic solids having high thermal and electrical conductivity. In a covalent network, the valence electrons are localized in covalent bonds, but in certain materials, light, heat, or an electrical field can excite the electrons and allow them to be conducted. Such materials are called semiconductors. 12.2 Electronic Structure of Materials Recall Molecular Orbital Theory – Atomic orbitals add to make molecular orbitals As the number of atoms bonded together within a molecule increases, the distance between the filled, bonding orbitals and unfilled, antibonding orbitals decreases. This energy difference between bonding and antibonding orbitals is called the band gap. Note that the band gap in a metal is virtually nonexistent, which results in high conduction. The band gap in a semiconductor is significant, but the gap can be influenced by a small bit of energy. The band gap in an insulator is too high to be consistently overcome, which is what causes insultation. 12.3 Semiconductors Elemental Semiconductors: One type of atom Ex. Silicon, germanium, gray tin, carbon as graphite (all but graphite adopt diamond’s crystal structure) Compound semiconductors: two or more elements Valence band – bonding molecular orbitals Conduction band – antibonding molecular orbitals Compound semiconductors maintain average of 4 valence electrons per atom Ex. GaAs (3 and 5), CdTe (2 and 6), and InP (3 and 5) Crystal structure of compound semiconductors is often face-centered, cubic structure, where each atom is surrounded by a tetrahedron of the opposite type of atom. Size of the band gap within a semiconductor depends on the position of the elements on the periodic table. Smaller elements will have increased orbital overlap, which leads to a larger splitting of the bonding and antibonding orbitals. Also, the larger the difference in electronegsativity between the atoms, the larger the band gap. Semiconductor Doping Doping with p-type semiconductors provide holes for electrons to move through Doping with n-type semiconductors provide extra electrons to hop into the p-type holes Alternating P-type and n-type semiconductors forms the basis of diodes, transistors, solar cells, and many other electronic devices 12.5 Superconductors Even metals are not infinitely conductive; there is some resistance to electron flow due to the vibrations of atoms and the presence of impurities and defects. In 1911, Dutch physicist H. Kamerlingh Onnes discovered that when mercury is cooled below 4.2 K, it loses all resistance to the flow of electrical current. This is known as superconductivity. Substances appear to have a superconducting transition temperature, below which the substance is a superconductor. 12.6 Polymers and Plastic Polymer – molecular substances of high molecular mass formed by the polymerization of monomers, molecules with low molecular mass. Plastics – materials that can be formed into various shapes, usually by the application of heat and pressure Thermoplastic – can be reshaped Thermoset plastic – cannot be reshaped easily Elastomer – a material that exhibits rubbery or elastic behavior Polymers are made by addition reactions (coupling monomers through their double bonds), condensation polymerization (removal of water), and other ways. Vulcanization of rubber: (a) is natural rubber, (b) is vulcanized rubber, where sulfur has been added to cross-link the polymer chains.