Rates of Reaction Lab Report: Temperature & Reaction Time
advertisement

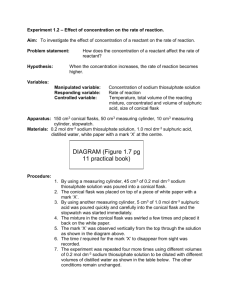
Afra Al Kuwari 9D Rates of Reaction Lab Report Research Question: How does the temperature of a solution affect the time it takes for different particles to collide? Aim: I am going to investigate the relationship between the time of a rate of reaction and the temperature of a solution by mixing different temperatures of Sodium Thiosulfate in Hydrochloric acid and time them. Hypothesis: I think that the larger the temperature of "Sodium Thiosulphate" the faster the reaction takes place. This is because when the mixture is heated, the molecules move much faster. As a result the particles will collide more often and with an increasing amount of energy. Variables: Independent Variables Temperature of sodium thiosulphate Dependant variables The time it takes for the reaction to occur Control Amount of Hydrochloric acid Amount of sodium thiosulphate Total Amount of Solution Fair Testing: For the experiment and testing to be fair I will make sure that the amount of "HCL" will always be 5ml, and the amount of "Sodium Thiosulphate" will be set at 50ml. Also, I will make sure that I will not use the same measuring cylinders for "HCL" and "Sodium Thiosulphate" will not get mixed up and they will both be different so the purity of the solution will stay and they will not get mixed up. Another point is that the "Blue" flame will be used throughout the whole experiment. All of these points will allow my final results to be more reliable and accurate and decrease the amount of errors, so this will lead the experiment to be more successful. Afra Al Kuwari 9D Materials: Stopwatch (1) Paper (for the ‘x’) HCL (80ml) Sodium thiosulphate (400ml) Diagram: Afra Al Kuwari 9D Method: 1. 2. 3. 4. 5. Gather all Materials Draw an "x" in the middle of the paper Turn the gas on and light the bunsen burner Turn the flame on the bunsen burner to blue Add 25 ml of "Sodium Thiosulphate" into the measuring cylinder and add it to the conical flask 6. Put the conical flask in the ice 7. Make sure the solution is as close to 10'C as possible, by using the thermometer 8. Place the conical flask on top of the "x", and make sure the "x" is at the center of the flask 9. Add 5ml of HCL to the solution and time how long it takes for the "x" to disappear 10. Keep it at room temperature 11. Repeat all the steps above 8 times but by adding 10 degrees each time but without using ice Results: Data Table: Temperature (C). 0 10 20 30 40 50 60 70 Time: Trial 1 2:06 48 20 13 10 4 3 Graph: (Attached) Afra Al Kuwari 9D Conclusion: The larger the temperature of sodium thiosulphate then the faster the molecules collide. When there’s a lot of heat, it gives energy, which makes the molecules vibrate faster each time I increase the temperature. When the temperature was 10 degrees Celsius, it took 2 minutes and 6 seconds for the solution to get completely cloudy. When the temperature was 70 degrees Celsius, it took only 3 seconds for the solution to get cloudy. These results show that my hypothesis was correct. Evaluation: We controlled the variables by adding the correct amount of HCL and also to have a balanced amount of sodium thiosulphate. To improve the lab, we can have different concentrations and amounts of HCL and sodium thiosulphate. The method was complicated when we did it because it took a lot of time to do. Also because we didn’t gather all the materials we needed from the beginning of the lab. To improve the method I would evenly distribute the ice in the beginning around the conical flask. I would also be more organized next time. The results on the graph are accurate to the results on the data table. When it took only a couple of seconds for the solution to get cloudy, the graph went down. The graph shows that the hypothesis was right and also explains the hypothesis.